Weak acid red bisazo dye and preparation method thereof
A technology of disazo dyes and acid red, which is applied in the direction of azo dyes, sulfonate preparation, dyeing methods, etc., can solve the problems that weak acid red dyes cannot be satisfied, and achieve low cost, good reproducibility, and excellent preparation process simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
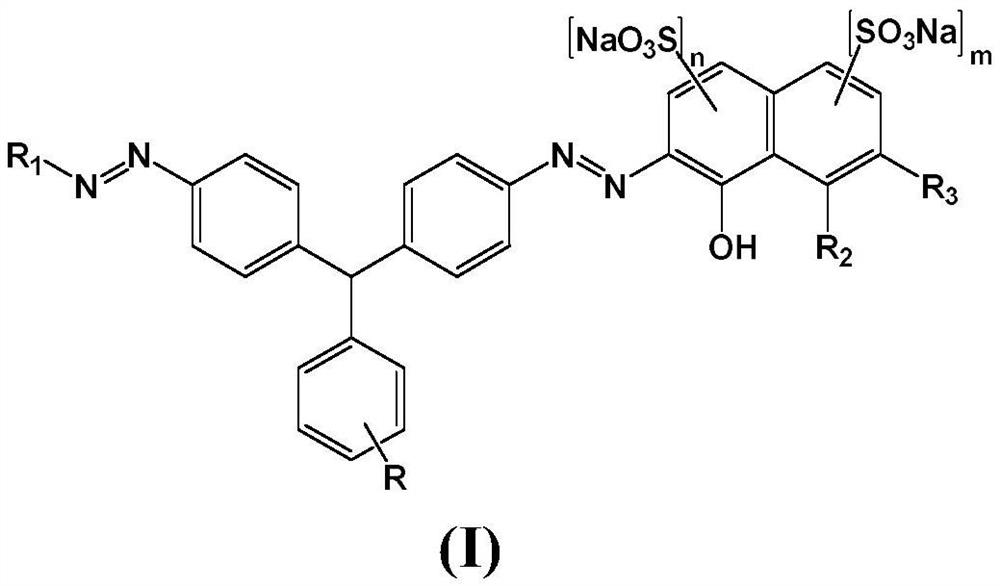
[0049] The present invention also provides a preparation method of the weak acid red disazo dye, comprising: diazotizing 4,4'-diaminotriphenylmethane compounds, and then performing a coupling reaction to obtain the weak acid red disazo dye .
[0050] In one embodiment, the preparation method of the present invention comprises:
[0051] Step 1: Diazotization of 4,4'-diaminotriphenylmethanes
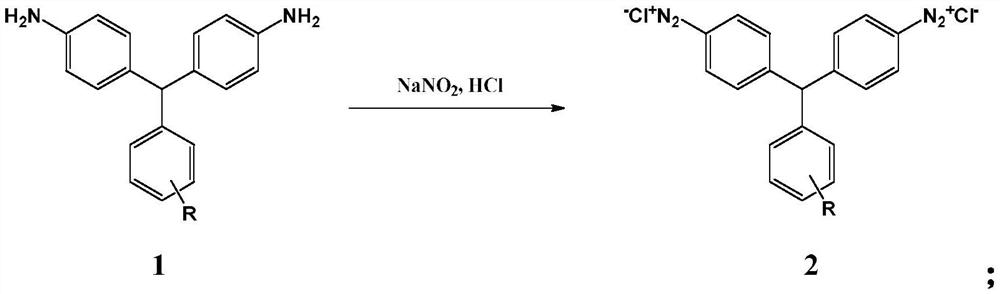
[0052] The 4,4'-diaminotriphenylmethane compound of formula 1 is diazotized in the presence of sodium nitrite and hydrochloric acid to obtain the diazonium salt of the 4,4'-diaminotriphenylmethane compound of formula 2
[0053]
[0054] Step 2: Coupling
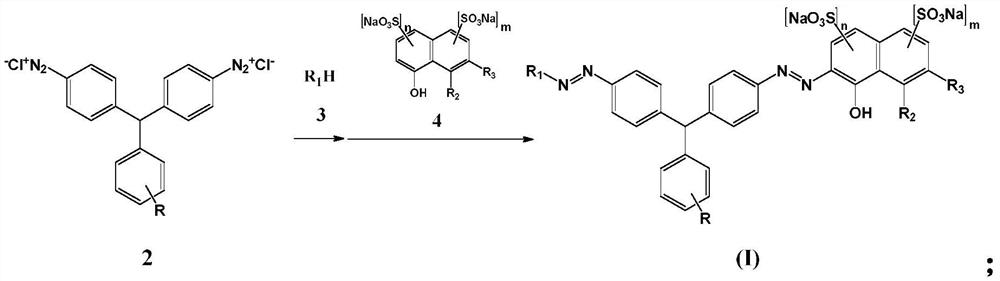
[0055] Under alkaline conditions, the diazonium salt of the 4,4'-diaminotriphenylmethane compound of formula 2 is first coupled with the compound of formula 3, and then coupled with the compound of formula 4 for the second time to obtain a solution of the coupled product
[0056]
[0057] Step 3: Postprocessing
[0058] The solu...
Embodiment 1
[0072] Embodiment 1: Weak acid red disazo dye A
[0073]
[0074] Step 1: Diazotization of 4,4'-diaminotriphenylmethane
[0075] Weigh 13.72g (0.05mol) of 4,4'-diaminotriphenylmethane, add 32ml (0.3mol) of 30% hydrochloric acid and 150ml of water, and stir to dissolve. Add ice to cool to 0°C, add 30% aqueous solution composed of 7g sodium nitrite (0.101mol) quickly and then slowly, react for 60 minutes, control pH=1.0 throughout the reaction process, and obtain 4,4'-diaminotri Benzyl diazonium salt, the test solution is blue with Congo red test paper, and the test solution is blue with starch potassium iodide test paper. After the reaction reaches the end, use sulfamic acid to eliminate excess nitrous acid, and filter to remove insoluble matter for later use.
[0076] Step 2: Coupling
[0077] Add 19.13g of sodium 2-naphthol-6,8-disulfonate (0.055mol) into 100ml of water, add 8g of soda ash to dissolve, and then adjust the pH to 7.5. Add 4,4'-diaminotriphenylmethane diaz...
Embodiment 2
[0083] Example 2: Weak acid red disazo dye E
[0084]
[0085] Step 1: Diazotization of 4,4'-diaminotriphenylmethane
[0086] Weigh 13.72g (0.05mol) of 4,4'-diaminotriphenylmethane, add 32ml (0.3mol) of 30% hydrochloric acid and 150ml of water, and stir to dissolve. Add ice to cool to 0°C, add 30% aqueous solution composed of 7g sodium nitrite (0.101mol) quickly and then slowly, react for 60 minutes, control pH=1.0 throughout the reaction process, and obtain 4,4'-diaminotri Benzyl diazonium salt, the test solution is blue with Congo red test paper, and the test solution is blue with starch potassium iodide test paper. After the reaction reaches the end, use sulfamic acid to eliminate excess nitrous acid, and filter to remove insoluble matter for later use.
[0087] Step 2: Coupling
[0088] Add 19.75g of 2,2-dimethyl-2,3-dihydro-1H-pithidine-5,8-disulfonic acid (0.055mol) into 100ml of water, add 16g of soda ash to dissolve, and then adjust the pH to 7.5. Add 4,4'-diamin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com