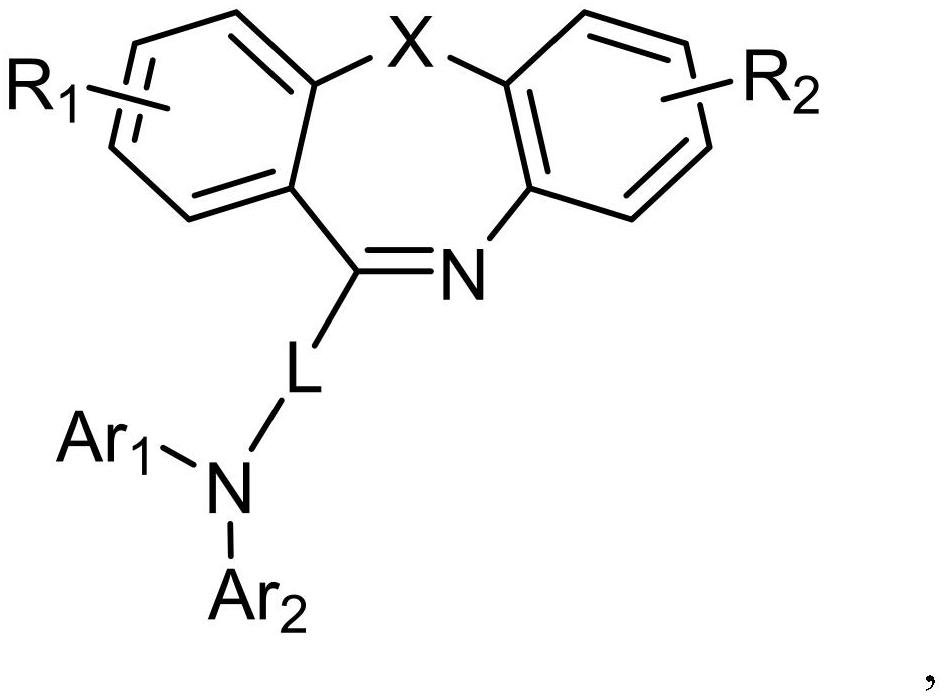
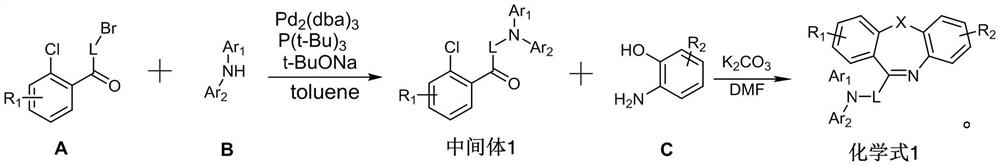
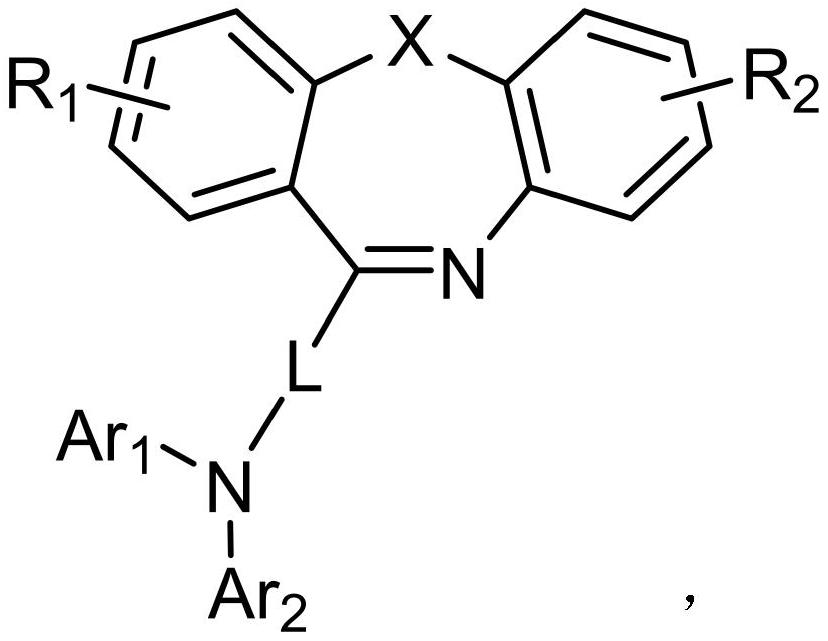
Dibenzo seven-membered heterocyclic compound and preparation method and application thereof
A seven-membered heterocycle and compound technology, which is applied in the preparation and application of luminescent materials, can solve the problems of reduced luminous efficiency and lifespan, complex synthesis process, and fast hole mobility, and achieves difficult aggregation, high hole mobility, high The effect of photothermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Synthesis of Compound-005
[0047] 1. Under nitrogen protection, dissolve raw material A-005 (30.00mmol) and raw material B-005 (30.00mmol) in 200.00ml of toluene solution, add tris(dibenzylideneacetone)dipalladium (0.3mmol), three tert-butylphosphine (1.5mmol) and sodium tert-butoxide (60.00mmol), stir evenly, raise the temperature to 90°C, and reflux for 5h; Salt and catalyst, after the filtrate was cooled to room temperature, washed three times with water, retained the organic phase, then extracted the aqueous phase with ethyl acetate; after combining the organic phases, dried using anhydrous magnesium sulfate (30.00g), and used a rotary evaporator Remove the solvent; use dichloromethane to dissolve the solid organic matter completely, then slowly add it dropwise to the petroleum ether solution, stir evenly, and a precipitate is precipitated, and the solid is obtained by suction filtration, rinsed with absolute ethanol and petroleum ether successively, and...
Embodiment 2
[0058] Example 2: Synthesis of Compound-059
[0059] 1. Under nitrogen protection, dissolve raw material A-059 (30.00mmol) and raw material B-059 (30.00mmol) in 250.00ml of toluene solution, add tris(dibenzylideneacetone)dipalladium (0.3mmol), three tert-butylphosphine (1.5mmol) and sodium tert-butoxide (60.00mmol), stir evenly, raise the temperature to 90°C, and reflux for 5h; salt and catalyst, after the filtrate was cooled to room temperature, washed three times with water, retained the organic phase, and then extracted the aqueous phase with ethyl acetate; after combining the organic phases, dried using anhydrous magnesium sulfate (35.00g), and used a rotary evaporator Remove the solvent; use dichloromethane to dissolve the solid organic matter completely, then slowly add it dropwise to the petroleum ether solution, stir evenly, and a precipitate is precipitated, and the solid is obtained by suction filtration, rinsed with absolute ethanol and petroleum ether successively,...
Embodiment 3
[0069] Example 3: Synthesis of Compound-069
[0070] 1. Under nitrogen protection, dissolve raw material A-069 (30.00mmol) and raw material B-069 (30.00mmol) in 200.00ml of toluene solution, add tris(dibenzylideneacetone)dipalladium (0.3mmol), three tert-butylphosphine (1.5mmol) and sodium tert-butoxide (60.00mmol), stir evenly, raise the temperature to 90°C, and reflux for 5h; Salt and catalyst, after the filtrate was cooled to room temperature, washed three times with water, retained the organic phase, then extracted the aqueous phase with ethyl acetate; after combining the organic phases, dried using anhydrous magnesium sulfate (30.00g), and used a rotary evaporator Remove the solvent; use dichloromethane to dissolve the solid organic matter completely, then slowly add it dropwise to the petroleum ether solution, stir evenly, and a precipitate is precipitated, and the solid is obtained by suction filtration, rinsed with absolute ethanol and petroleum ether successively, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com