HER2 protein targeting polypeptide and application thereof
A protein-targeted technology, applied in the field of biomedicine, can solve the problems of dyspnea, liver and kidney function decline, drug resistance in patients, etc., and achieve the effect of high throughput and high screening efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1O
[0070] The establishment of embodiment 1 OBOC polypeptide library
[0071] In this embodiment, the OBOC method is used to synthesize a polypeptide library on resin beads (beads), and the steps are as follows:
[0072] (1) Take a certain amount of polypeptide synthetic resin and place it in a synthetic tube, add a certain amount of DMF to immerse the resin to swell the resin, and soak for more than 2 hours;
[0073] (2) Add Fmoc-protected methionine (M) to the synthesis tube, and react on a shaking table for more than 2 hours; alternately wash three times with methanol and DMF, and repeatedly blow up the resin with a straw during the washing process; then carry out ninhydrin Boiling water bath test, resin color is colorless;
[0074] (3) Add deprotection agent to carry out Fmoc deprotection for 10min, alternately rinse with methanol and DMF three times, and repeatedly blow up the resin with a straw during the rinse process; repeat deprotection once, that is, two consecutive de...
Embodiment 2
[0081] FITC labeling of embodiment 2 HER2 protein
[0082] In this example, the HER2 protein (SEQ ID NO: 12) is labeled with FITC, and the steps are as follows:
[0083] (1) Dissolve HER2 protein in PBS so that the final concentration of HER2 protein is 10-50 μg / mL;
[0084] (2) Add FITC to the HER2 protein solution prepared in step (1), so that the amount of FITC-labeled protein is 5%, place in an ice bath, and magnetically stir overnight under dark conditions;
[0085] (3) The next day, place the mixed solution in a dialysis bag with a molecular weight cut-off of 500, and dialyze for three days under deionized water conditions;
[0086] (4) The dialyzed solution is collected and freeze-dried by a freeze dryer to obtain FITC-labeled HER2 protein.
Embodiment 3
[0087] Example 3 Screening of polypeptides targeting HER2 protein
[0088] Incubate the FITC-labeled HER2 protein and the peptide library in PBS buffer for 2 hours, observe the color of the peptide resin excited by a wavelength of 405 nm under a fluorescence microscope, and select the observed peptide resin with obvious green fluorescence;
[0089] Use 30% CNBr for the selected peptide resin 3 The ethanol solution was lysed overnight, the supernatant was obtained by centrifugation, and the peptide sequencing was carried out after desalting treatment.
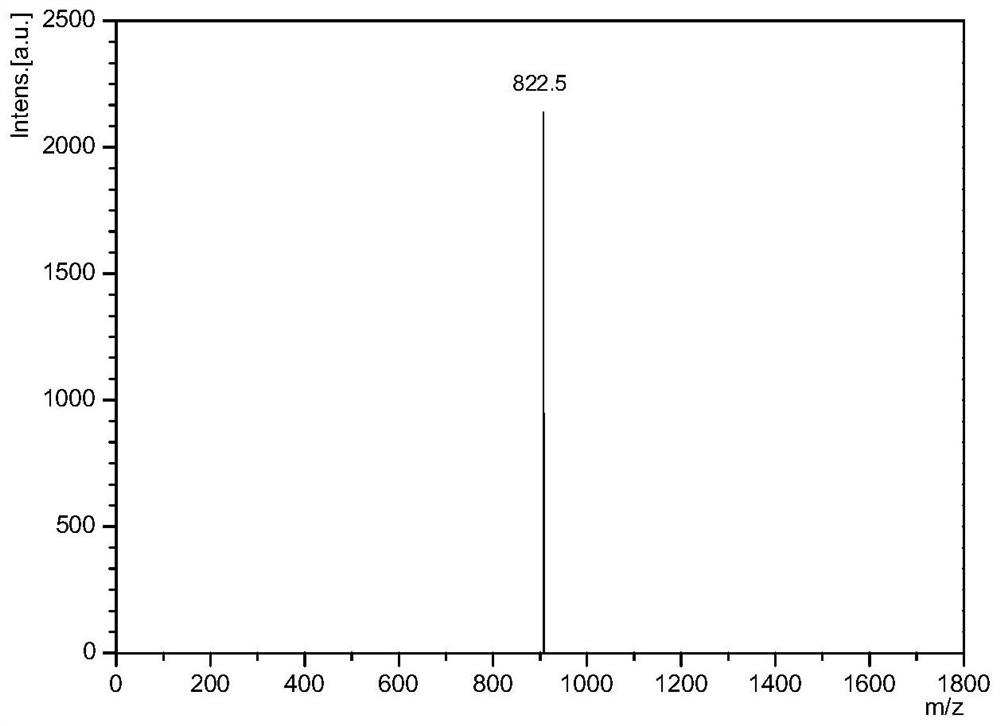
[0090] figure 1 Shown is the primary mass spectrogram of the polypeptide sequence, and the measured molecular weight of the polypeptide is close to the theoretical molecular weight, which is the desired polypeptide sequence.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com