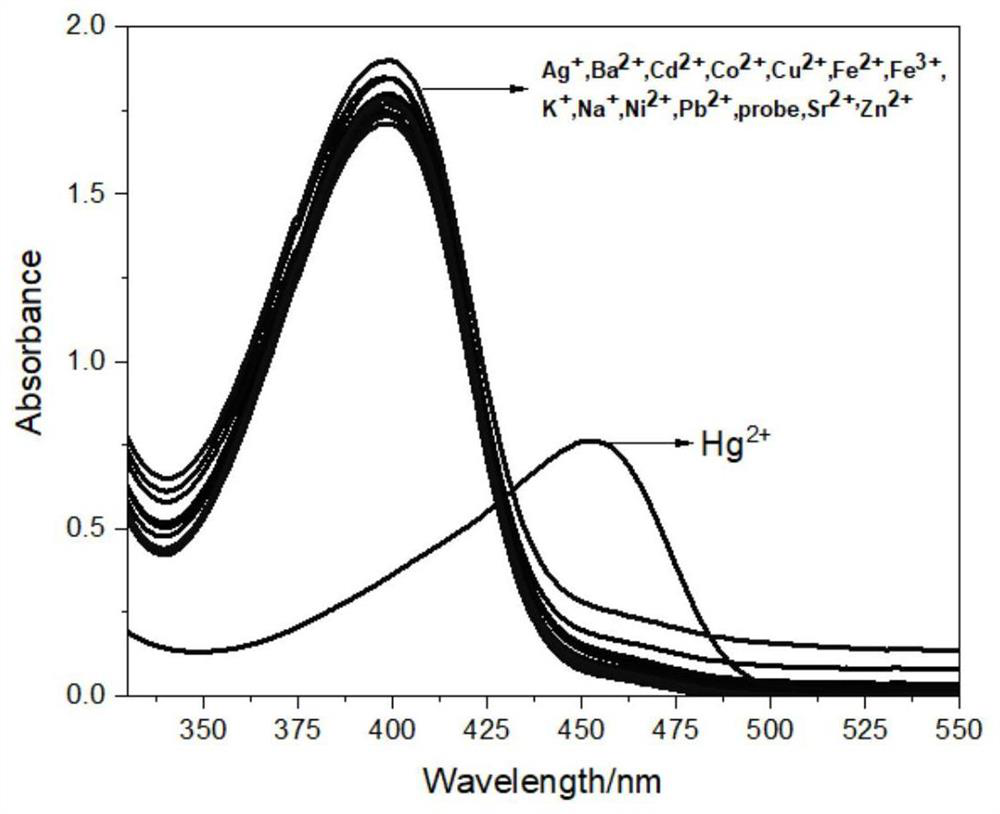
Coumarin derivative-based fluorescent probe and preparation method thereof
A technology of coumarin derivatives and fluorescent probes, applied in the field of fluorescent probes, can solve the problems of expensive operation and complexity of instruments, and achieve the effect of good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
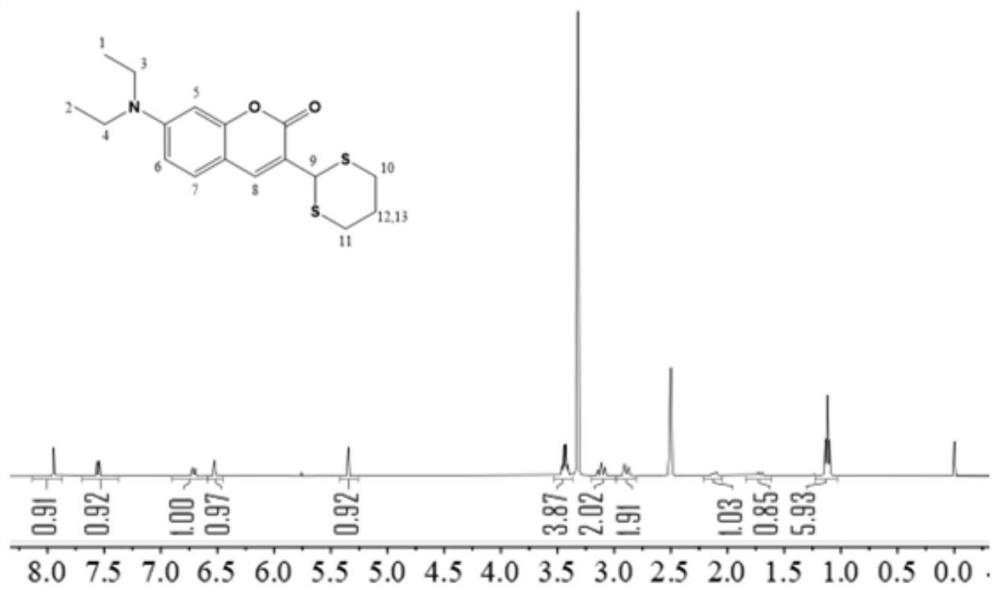
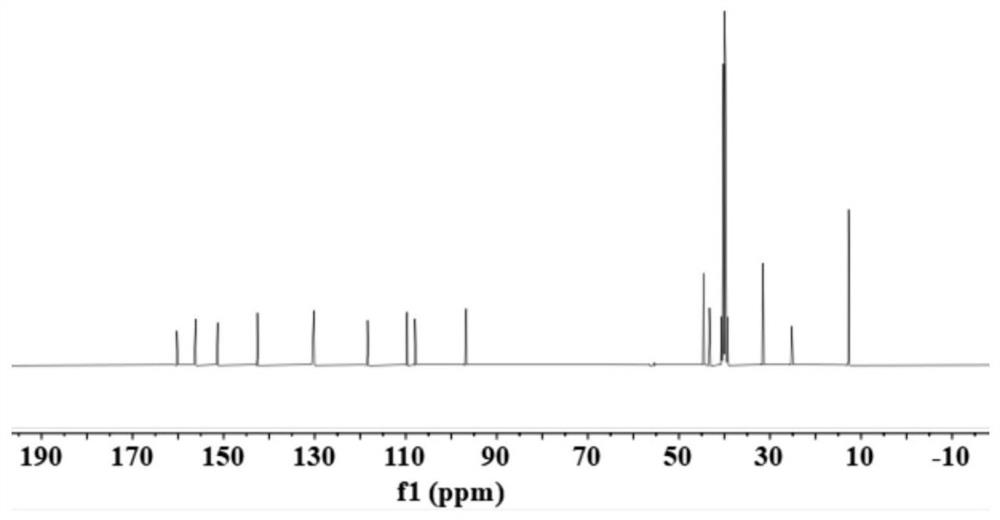
[0034] Weigh 1.93g of 4-(diethylamino)salicylaldehyde, 3ml of diethyl malonate, and 1ml of piperidine, dissolve in 60ml of absolute ethanol, add 2 drops of glacial acetic acid, pre-dissolve at room temperature, and heat in an oil bath , reflux, the temperature rose to 85 ° C, reacted at a constant temperature for 24 hours, cooled to room temperature, and the solvent was removed by rotary evaporation. Add 20ml of concentrated hydrochloric acid and 20ml of glacial acetic acid to the swirled solution, and react at 100°C for 6h. Cool to room temperature, adjust pH to 5 with 30% NaOH solution, stir for 1 h, filter, wash with water 3 times, and dry to obtain the crude product. The crude product was separated and purified by column chromatography to obtain 7-(diethylamino)coumarin, namely compound 1.
[0035] Weigh 2.25g compound 1, dissolve it with 15ml DMF, and dissolve 6.5ml POCl 3 Add to 6.5ml DMF, mix well, and use the dropping funnel to add POCl 3 The mixed solution with DMF...
Embodiment 2
[0038] Weigh 1.93g of 4-(diethylamino)salicylaldehyde, 3ml of diethyl malonate, and 1ml of piperidine, dissolve in 60ml of absolute ethanol, add 2 drops of glacial acetic acid, pre-dissolve at room temperature, and heat in an oil bath , reflux, the temperature rose to 85 ° C, reacted at a constant temperature for 24 hours, cooled to room temperature, and the solvent was removed by rotary evaporation. Add 20ml of concentrated hydrochloric acid and 20ml of glacial acetic acid to the swirled solution, and react at 90°C for 7h. Cool to room temperature, adjust the pH to 5 with 30% NaOH solution, stir for 1 h, filter, wash with water 3 times, and dry the crude product. The crude product was separated and purified by column chromatography to obtain 7-(diethylamino)coumarin, namely compound 1.
[0039] Weigh 2.25g compound 1, dissolve it with 15ml DMF, and dissolve 6.5ml POCl 3 Add to 6.5ml DMF, mix well, and use the dropping funnel to add POCl 3 The mixed solution with DMF was ad...
Embodiment 3
[0042] Weigh 1.93g of 4-(diethylamino)salicylaldehyde, 3ml of diethyl malonate, and 1ml of piperidine, dissolve in 60ml of absolute ethanol, add 2 drops of glacial acetic acid, pre-dissolve at room temperature, and heat in an oil bath , reflux, the temperature rose to 85 ° C, reacted at a constant temperature for 24 hours, cooled to room temperature, and the solvent was removed by rotary evaporation. Add 20ml of concentrated hydrochloric acid and 20ml of glacial acetic acid to the swirled solution, and react at 110°C for 6h. Cool to room temperature, adjust pH to 5 with 30% NaOH solution, stir for 1 h, filter, wash with water 3 times, and dry to obtain the crude product. The crude product was separated and purified by column chromatography to obtain 7-(diethylamino)coumarin, namely compound 1.
[0043] Weigh 2.25g compound 1, dissolve it with 15ml DMF, and dissolve 6.5ml POCl 3 Add to 6.5ml DMF, mix well, and use the dropping funnel to add POCl 3 The mixed solution with DMF...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com