Desorption method based on adsorption of calcined bone apatite to heavy metal ions
A technology of heavy metal ions and bone apatite, applied in separation methods, chemical instruments and methods, adsorption water/sewage treatment, etc., can solve the problems of inability to carry out secondary adsorption, improve cycle regeneration performance, reduce acidity, and cost cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] First, weigh 14.612g of ethylenediaminetetraacetic acid with a balance and dissolve it in a 1L beaker, seal the beaker with plastic wrap (to avoid the increase in concentration caused by the evaporation of heating water vapor), place it on a magnetic stirrer, heat to 80°C and stir for 12h to After the EDTA is completely melted, prepare an EDTA solution with a concentration of 0.05mol / L, then take 100mL of an EDTA aqueous solution with a concentration of 0.05mol / L and dilute it to 0.001mol / L, and then add Saturated calcium dihydrogen phosphate, configured as a pH=5 mixed solution, used as a desorption solution for osteogenic apatite to adsorb heavy metal ions (Cu, Zn, Pb, Cd).
[0061] Below with Pb 2+ The desorption process as an example:
[0062] 1 g of calcined bone apatite was placed in 50 mL of Pb 2+ Concentration is in the beaker of 200mg / L, and the beaker is sealed with plastic wrap, is placed in the vibration shaker and oscillates and absorbs for 24h. It can b...
Embodiment 2
[0068] First, weigh 14.612g of ethylenediaminetetraacetic acid with a balance and dissolve it in a 1L beaker, seal the beaker with plastic wrap (to avoid the increase in concentration caused by the evaporation of heating water vapor), place it on a magnetic stirrer, heat to 80°C and stir for 12h to After the EDTA is completely melted, it is prepared into an EDTA solution with a concentration of 0.05mol / L, and then saturated calcium dihydrogen phosphate is added to form a mixed solution with pH=3, which is used as a bone-forming apatite to adsorb heavy metal ions (Cu, Zn, Pb, Cd) desorption solution.
[0069] Below with Pb 2+ The desorption process as an example:
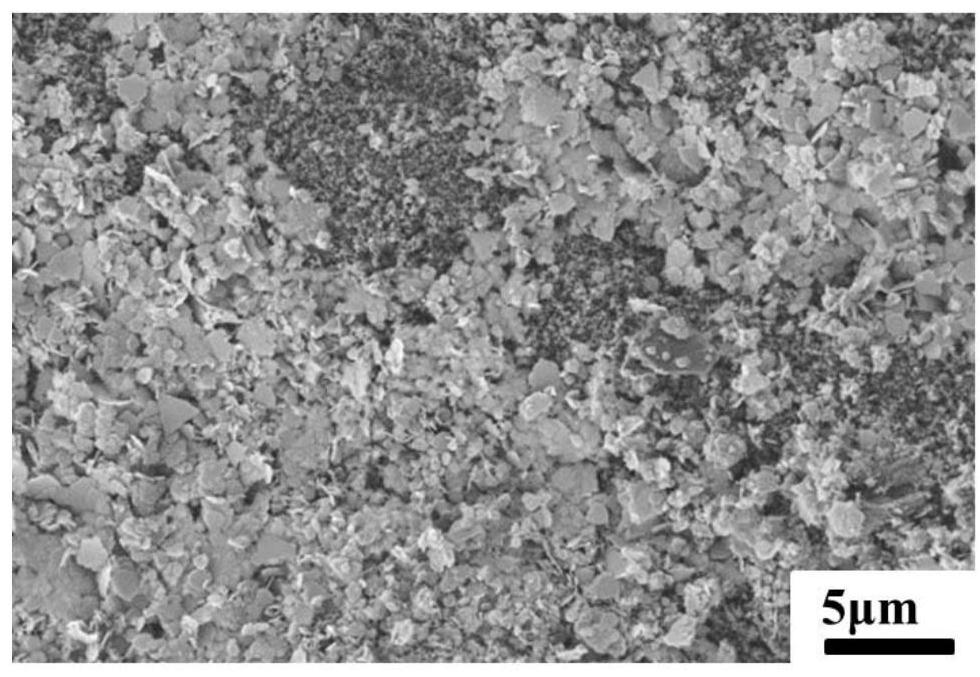
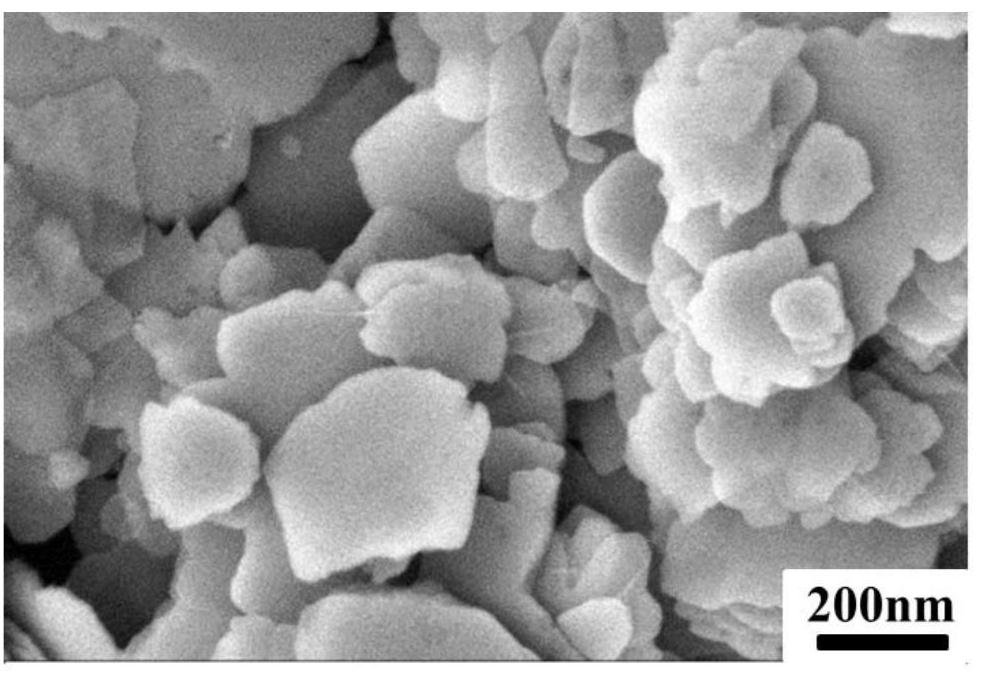
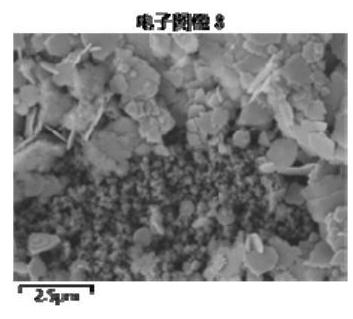
[0070] 1 g of calcined bone apatite was placed in 50 mL of Pb 2+ Concentration is in the beaker of 200mg / L, and the beaker is sealed with plastic wrap, is placed in the vibration shaker and oscillates and absorbs for 24h. It can be seen from Figure 1(a) and Figure 1(b) that it is mainly in the shape of a sheet, wi...
Embodiment 3
[0076] First, weigh 14.612g of ethylenediaminetetraacetic acid with a balance and dissolve it in a 1L beaker, seal the beaker with plastic wrap (to avoid the increase in concentration caused by the evaporation of heating water vapor), place it on a magnetic stirrer, heat to 80°C and stir for 12h to After the EDTA is completely melted, prepare an EDTA aqueous solution with a concentration of 0.05mol / L, then take 100mL of an EDTA aqueous solution with a concentration of 0.05mol / L and dilute it to 0.005mol / L, and then add Saturated calcium dihydrogen phosphate, configured as a pH=4 mixed solution, used as a desorption solution for bone apatite to adsorb heavy metal ions (Cu, Zn, Pb, Cd).
[0077] Below with Pb 2+ The desorption process as an example:
[0078] 1 g of calcined bone apatite was placed in 50 mL of Pb 2+ Concentration is in the beaker of 200mg / L, and the beaker is sealed with plastic wrap, is placed in the vibration shaker and oscillates and absorbs for 24h. It can...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com