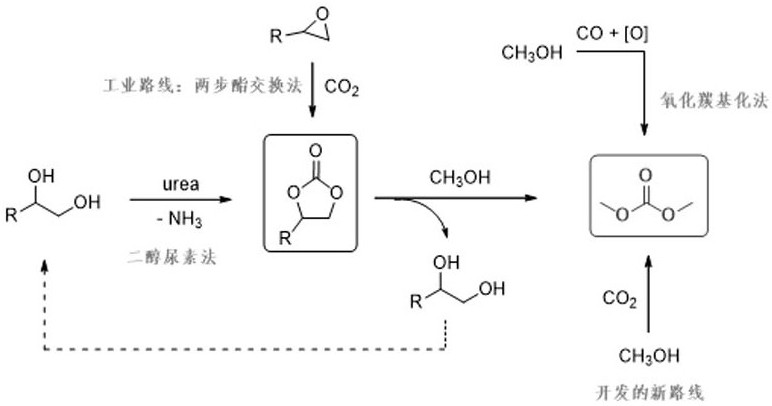
Catalyst for preparing dimethyl carbonate from methanol, carbon dioxide and 2-cyanopyridine and its preparation method and application
A technology of dimethyl carbonate and cyanopyridine, which is applied in the field of preparation of dimethyl carbonate, can solve the problems of difficult separation and purification of products, low recycling efficiency, consumption of dehydrating agent, etc., to facilitate large-scale production, increase production capacity, and reduce costs low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Prepare the concentration of Cr ion to be 0.1 g·mL -1 6 mL of chromium nitrate solution, and γ-Al with a particle size of 20 nm was added to the solution 2 o 3 The solid was 8.9 g, and stirred evenly, then 10 mL of H3BO3 solution with a concentration of 0.9 mol / L was added, stirred again to mix evenly, soaked for 6 h, then dried at 100 °C for 12 h, and the obtained solid was ground, 400 °C Calcined under the same conditions for 6 h to obtain the catalyst. Take 10 g of catalyst raw powder and fill it into the tank reactor. Methanol and 2-cyanopyridine are mixed and miscible at a molar ratio of 3:1 and then fed in liquid form. The liquid material accounts for 12% of the reactor volume. CO2 is fed in a gaseous state, and CO2 is charged to 1 MPa. The reaction temperature was 100 °C, the mass ratio of catalyst to methanol was 0.06, the residence time of the reactants in the reaction bed was 8 h, the conversion rate of methanol was 88.7%, the selectivity of DMC was 100%, a...
Embodiment 2
[0030] Prepare Zr ion concentration to be 0.07 g·mL -1 10 mL of zirconium nitrate solution, and SiO with a particle size of 20 nm was added to the solution 2 Solid 8.95 g, and stir evenly, then add the H that concentration is 0.45 mol / L 3 BO 3 10 mL of the solution was stirred and mixed evenly, impregnated for 6 h, then dried at 100 °C for 16 h, the obtained solid was ground, and calcined at 400 °C for 10 h to obtain the catalyst. Take 10 g of catalyst raw powder and fill it into the tank reactor. Methanol and 2-cyanopyridine are mixed and miscible at a molar ratio of 1:1 and then fed in liquid form. The liquid material accounts for 25% of the reactor volume. CO2 is fed in a gaseous state, and CO2 is charged to 5 MPa. The reaction temperature was 120 °C, the mass ratio of catalyst to methanol was 0.05, the residence time of the reactants in the reaction bed was 8 h, the methanol conversion rate was 91.2%, the DMC selectivity was 100%, and the number of catalyst cycles was ...
Embodiment 3
[0032] Prepare the concentration of Mn ion to be 0.15 g·mL -1 10 mL of manganese nitrate solution, the concentration of Ni ions is 0.08 g·mL -1 10 mL of nickel nitrate solution, and γ-Al with a particle size of 50 nm was added to the mixed solution 2 o 3 Solid 16.6 g, and stir evenly, then add the H that concentration is 0.7 mol / L 3 20 mL of PO4 solution was stirred and mixed evenly again, impregnated for 8 h, then dried at 120 °C for 8 h, the obtained solid was ground, and calcined at 500 °C for 4 h to obtain the catalyst. Take 10 g of catalyst raw powder and fill it into the tank reactor. Methanol and 2-cyanopyridine are mixed and miscible at a molar ratio of 1:2 and then fed in liquid form. The liquid material accounts for 25% of the reactor volume, and the CO 2 Feed in gaseous state, filled with CO 2to 3 MPa, the reaction temperature is 115°C, the mass ratio of catalyst to methanol is 0.1, the residence time of the reactants in the reactor is 6 h, the methanol convers...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com