A kind of insecticidal and antibacterial 5-imino substituted derivatives and its preparation method and application
A derivative and imino technology, applied in the field of 5-imino substituted derivatives and preparation, to achieve the effect of convenient synthesis method, strong market competitiveness, and rich structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
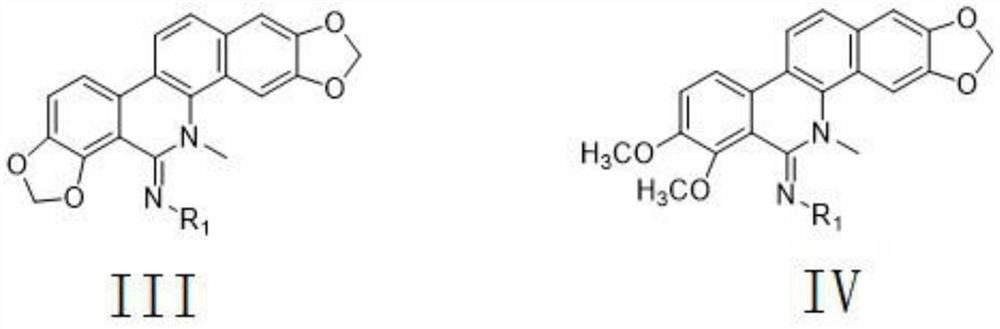
[0069] A 5-imino substituted derivative with insecticidal and antibacterial properties, the 5-imino substituted derivative is a polysubstituted-1H-1,2,4-triazole amidine compound, and its general structural formula is:
[0070]
[0071] Further, R in the general structural formula 1 Selected from H, the general formula is C n H 2n+1 or C m H 2m-1 straight or branched chain aliphatic hydrocarbon group, substituted or unsubstituted phenyl group, substituted or unsubstituted benzyl group, substituted or unsubstituted pyridyl group, substituted or unsubstituted furanyl group, substituted or unsubstituted triazolyl group, Wherein, n is an integer of 1-18, m is an integer of 3-13; R in the general structural formula 1 One or more of substituted or unsubstituted 1,2,4-triazolyl, chloropyridyl, and chlorothiazolyl; the substituents are F, Cl, Br, I, hydroxyl, C1 One or more of -4 alkyl groups, C1-4 alkoxy groups, trifluoromethyl groups, amino groups, nitro groups, cyano groups...
Embodiment 2
[0083] A 5-imino substituted derivative with insecticidal and antibacterial properties, the 5-imino substituted derivative is a polysubstituted-1H-1,2,4-triazole amidine compound, and its general structural formula is:
[0084]
[0085] Further, R in the general structural formula is selected from H, the general formula is C n H 2n+1 or C m H 2m-1 straight or branched chain aliphatic hydrocarbon group, substituted or unsubstituted phenyl group, substituted or unsubstituted benzyl group, substituted or unsubstituted pyridyl group, substituted or unsubstituted furanyl group, substituted or unsubstituted triazolyl group, Wherein, n is an integer of 1-18, m is an integer of 3-13; R1 in the general structural formula is one of substituted or unsubstituted 1,2,4-triazolyl, chloropyridyl, and chlorothiazolyl one or more of ; the substituents are F, Cl, Br, I, hydroxyl, C1-4 alkyl, C1-4 alkoxy, trifluoromethyl, amino, nitro, cyano, carboxyl , one or more of propionyloxy, acetox...
Embodiment 3
[0097] A 5-imino substituted derivative with insecticidal and antibacterial properties, the 5-imino substituted derivative is a polysubstituted-1H-1,2,4-triazole amidine compound, and its general structural formula is:
[0098]
[0099] Further, R in the general structural formula is selected from H, the general formula is C n H 2n+1 or C m H 2m-1 straight or branched chain aliphatic hydrocarbon group, substituted or unsubstituted phenyl group, substituted or unsubstituted benzyl group, substituted or unsubstituted pyridyl group, substituted or unsubstituted furanyl group, substituted or unsubstituted triazolyl group, Wherein, n is an integer of 1-18, m is an integer of 3-13; R1 in the general structural formula is one of substituted or unsubstituted 1,2,4-triazolyl, chloropyridyl, and chlorothiazolyl one or more of ; the substituents are F, Cl, Br, I, hydroxyl, C1-4 alkyl, C1-4 alkoxy, trifluoromethyl, amino, nitro, cyano, carboxyl , one or more of propionyloxy, acetox...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com