Application of bacteroides fragilis in preparation of medicine for treating inflammatory bowel diseases
A technology for Bacteroides fragilis and inflammatory bowel disease, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Cultivation of Bacteroides fragilis ATCC 25285
[0026] 1. Training method
[0027] TSA + 10% sterile defibrinated sheep blood, cultured for 48 hours at 37°C under strict anaerobic conditions.
[0028] The formula of 1L TSA medium is as follows: tryptone...15g
[0029] Soy peptone...5.0g
[0030] NaCl……………5.0g
[0032] .
[0033] Weigh 40g of this product, dissolve it in 1000ml of distilled water with heating and stirring, divide into Erlenmeyer flasks, and autoclave at 121°C for 15 minutes. Cool to about 50 degrees, add 10% sterile defibrinated sheep blood, shake and mix well, pour plate. Spread the bacteria on the plate and culture according to the condition.
Embodiment 2
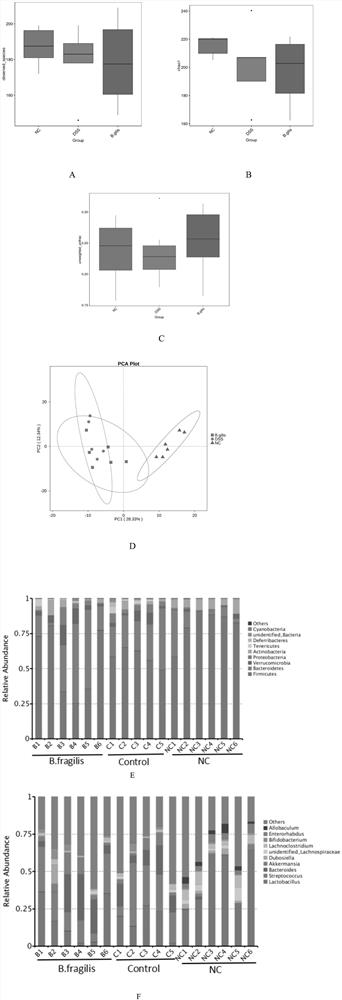
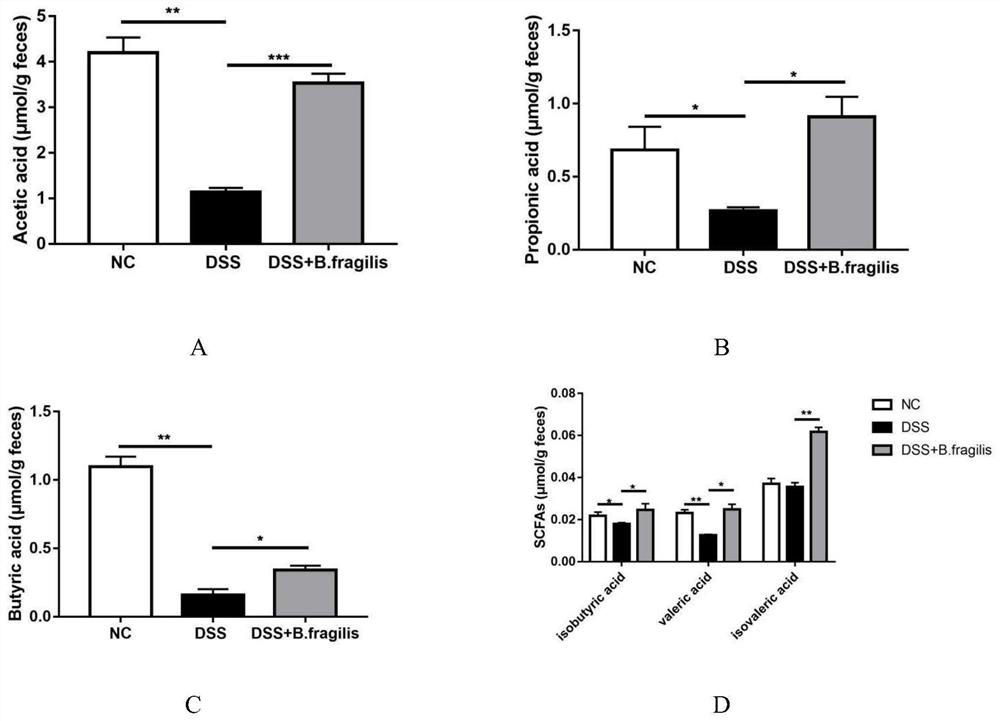
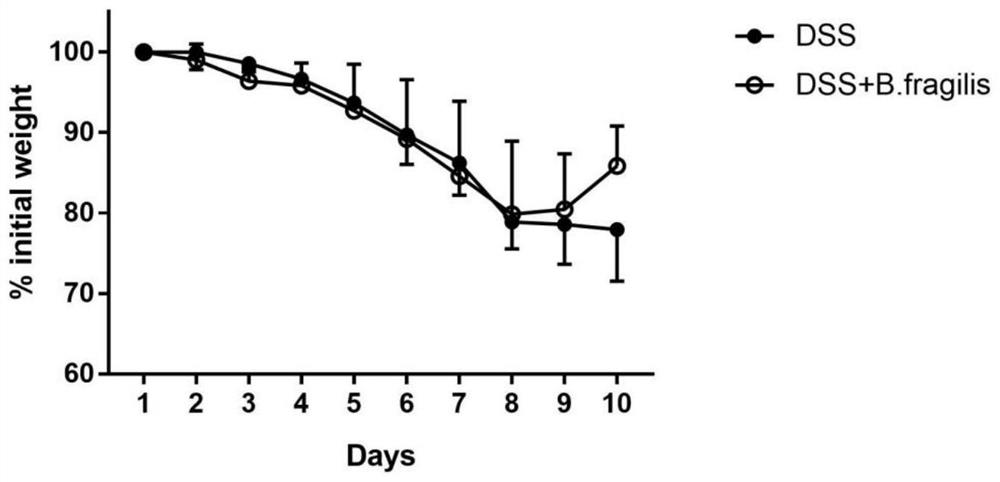
[0035] Effect of Bacteroides fragilis ATCC 25285 in the treatment of colitis
[0036] Select healthy and mature SPF grade C57 / BL6 female mice, weighing 18-22g, purchased from Nanjing Qinglongshan Animal Breeding Farm, a total of 18 mice were randomly divided into 3 groups, 6 mice in each group, and all mice were adaptively fed for 1 day before the experiment. week.
[0037] 1. Grouping and administration of animals
[0038] Blank control group (NC group): Mice drank sterile water.
[0039] Colitis group (DSS group): the mice were given 3% DSS solution as drinking water, and after 7 days, they were replaced with normal drinking water, and 200 μL of sterile water was fed into the stomach every day, and they were killed after 3 days.
[0040] Administration group (DSS+B.fragilis group): the mice were given 3% DSS solution as drinking water, changed to normal drinking water after 7 days, and 200 μL of Bacteroides fragilis (containing live bacteria 10 6 -10 10 CFU), sacrificed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com