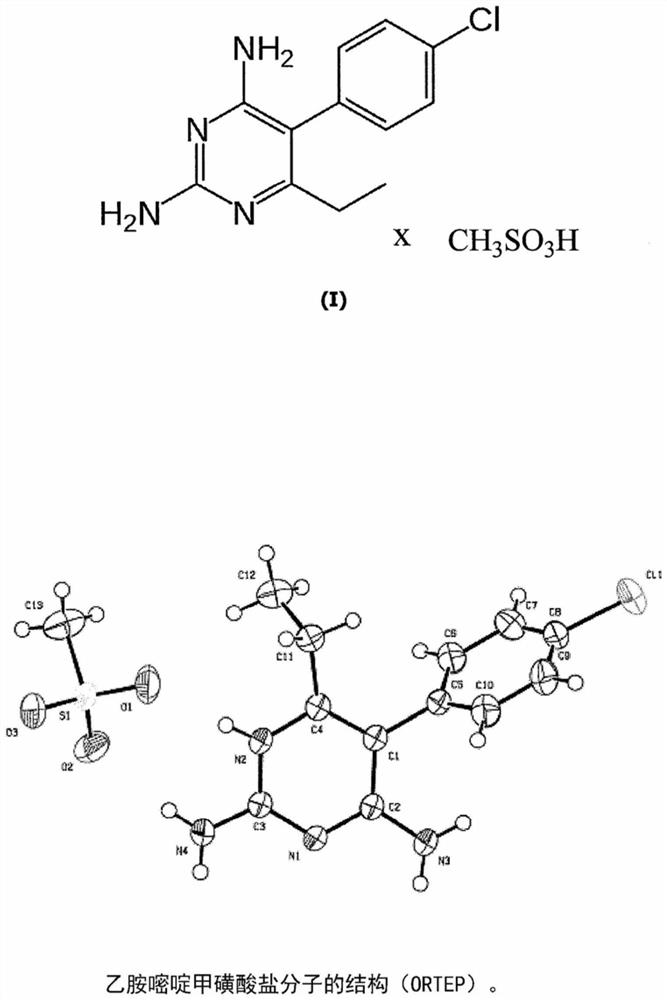
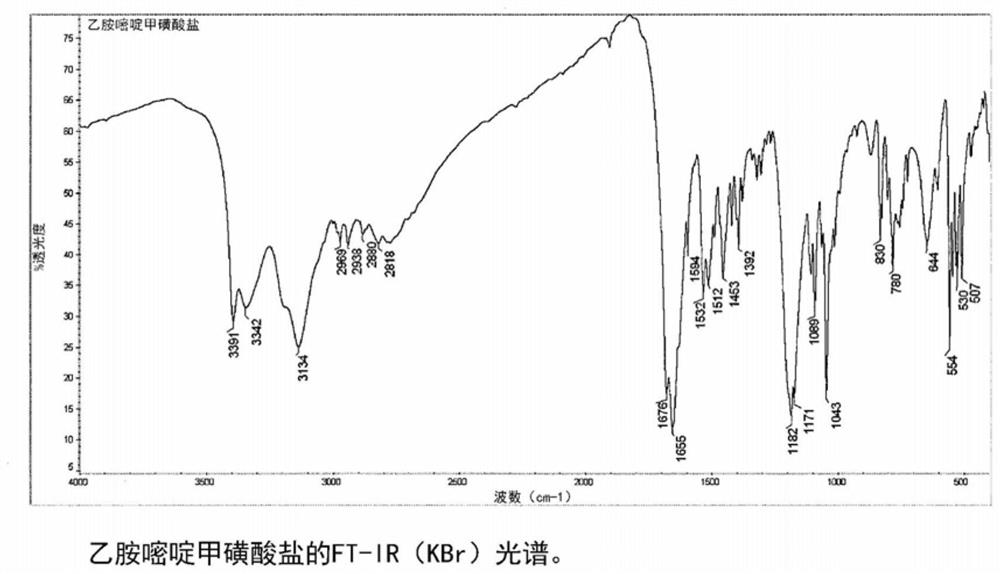
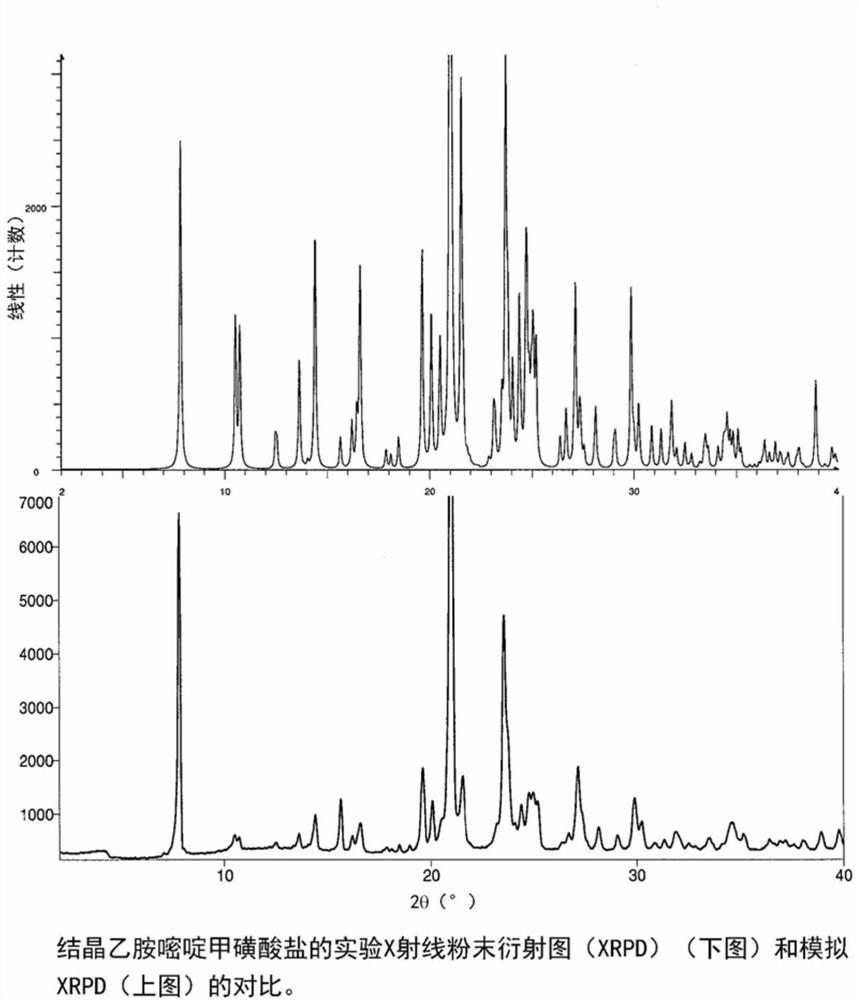
Acid addition salt of pyrimethamine
A technology of acid addition salt and pyrimethamine base, applied in the direction of organic active ingredients, resistance to vector-borne diseases, medical preparations containing active ingredients, etc., can solve problems such as solubility without mentioning
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Pyrimethamine (30g) (purity 99.83%, containing 0.16% impurity, R f =2.53) was crystallized in ethylene glycol (3 x 60 mL). The obtained crystals were refluxed in ethanol (50 mL), filtered and dried at 60°C under reduced pressure. 25 g of pyrimethamine containing 0.09% R f= 2.53 impurities. The obtained product (24.9 g, 0.1 mol) was suspended in acetone (250 mL), and methanesulfonic acid (24.0 g, 0.25 mol) was added to the suspension. The reaction was stirred at room temperature for 60 minutes, then the white precipitate was collected and washed with acetone (500 mL). The product was suspended in ethanol (250 mL) and refluxed with stirring for 15 minutes. Then, the suspension was cooled to room temperature, and the precipitate was collected, washed with ethanol (250 mL), and dried at 60° C. under reduced pressure (20 mmHg). The product was obtained as white crystals in a yield of 24.0 g (70%). The acid content of the salt was determined by potentiometric titration:...
Embodiment 2
[0100] Pyrimethamine (24.9 g, 0.1 mol) was dissolved in hot ethylene glycol (100 mL), methanesulfonic acid (24.0 g, 0.25 mol) was added to the solution, and the reaction mixture was cooled to room temperature. Then, acetone (200 mL) was added, and the mixture was left at room temperature for 12 hours. The precipitate was collected, washed with acetone (50 mL), and dried at 60° C. under reduced pressure (20 mm Hg). The product was obtained as white crystals in a yield of 18.0 g (52%). The acid content of the salt was determined by potentiometric titration: 28.20% (calculated: 27.87%).
Embodiment 3
[0102] Pyrimethamine (249 g, 1 mol) was suspended in 96% ethanol (1 L), and methanesulfonic acid (240 g = 162 mL, 2.5 mol) was added. The reaction mixture was stirred at room temperature for 60 minutes. Then reflux for 30 minutes. After cooling, the white precipitate was collected and washed with ethanol (500 mL). The product was suspended in ethanol (1 L) and refluxed for 15 minutes with stirring. The mixture was then cooled to room temperature and the precipitate was collected and washed sequentially with ethanol (200 mL), acetone (1 L) and again with ethanol 10 (200 mL). The product was dried at 60°C under reduced pressure (20 mm Hg) to give white crystals in a yield of 278 g (81%). Acid content: 27.92% (calculated: 27.87%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com