A kind of preparation method of olaparib
A piperazine and carbonyl technology, applied in the field of preparation of olaparib, can solve the problems of high activity of cyclopropylcarbonyl chloride, low yield of olaparib, complicated purification steps, etc., and achieves easy industrial scale-up production, method Simple, chromatographically pure results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
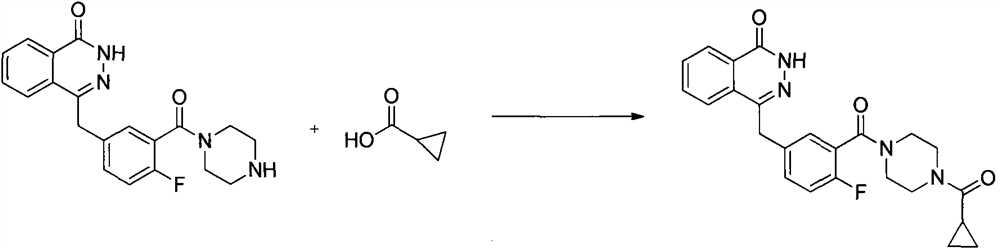
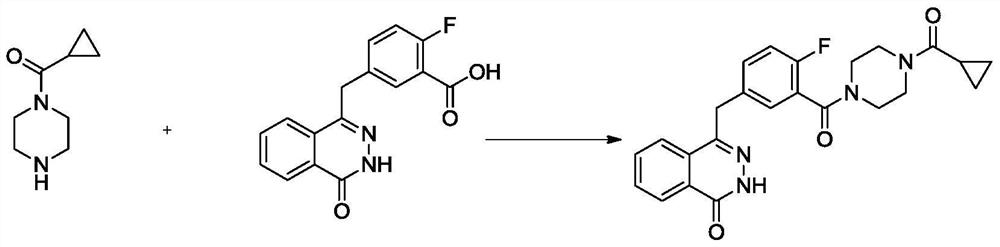
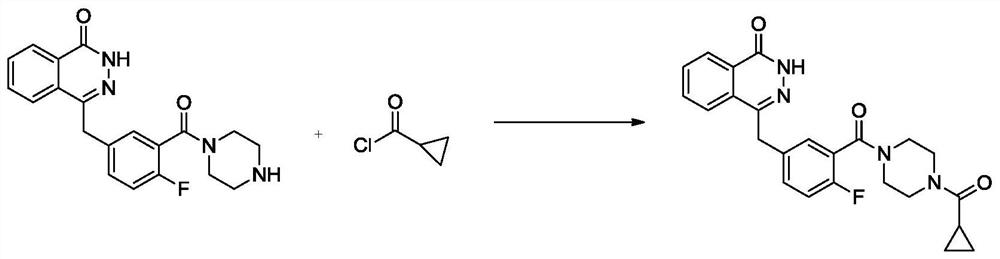
[0037] At 10°C, add 2000ml of N,N-dimethylacetamide to the reaction kettle, and add 4-(4-fluoro-3-(piperazine-1-carbonyl)benzyl)phthalein-1( 2-Hydro)-ketone (40g, 0.109mol), HBTU (48g, 0.127mol), cyclopropanecarboxylic acid (12g, 0.139mol), and finally N,N-diisopropylethylamine (0.218mol, 28.17g , 38ml), after reacting at 10°C for 8 hours, add 800ml of water, slowly precipitate the solid, suction filter, wash, and dry to obtain 42g of white solid, namely olaparib; HPLC purity 99.83%, yield 88.6%.
[0038] NMR data of olaparib:
[0039] 1 H NMR (400Hz, DMSO) δ12.598(s, 1H), 8.26(dd J=10.0Hz, 1.4Hz, 1H), 7.96(d J=10.0Hz, 1H), 7.90(d J=8.2Hz, 1H ),7.80-7.86(m,1H),7.42-7.46(m,1H),7.38(d J=7.7Hz,1H),7.23(d J=12.2Hz,1H),4.33(s,2H),3.41 -3.74 (m, 6H), 3.19 (s, 2H), 1.94 (dJ=22.7Hz, 1H), 0.70-0.75 (m, 4H).
[0040] 13 C NMR (100Hz, DMSO) δ171.2, 164.0, 159.3, 158.0, 154.7, 144.7, 134.8, 134.7, 133.4, 131.7, 131.6, 131.4, 129.0, 128.93, 128.88, 127.9, 126.0, 1047.3, 12...
Embodiment 2
[0042]
[0043] At 50°C, add 2000ml of N,N-dimethylacetamide into the reaction kettle, and add 4-(4-fluoro-3-(piperazine-1-carbonyl)benzyl)phthalein-1( 2-Hydro)-ketone (40g, 0.109mol), HBTU (48g, 0.127mol), cyclopropanecarboxylic acid (12g, 0.139mol), and finally N,N-diisopropylethylamine (0.218mol, 28.17g , 38ml), reacted at 50°C for 4 hours, cooled to room temperature, added 800ml of water, slowly precipitated solid, suction filtered, washed, and dried to obtain 39g of white solid, namely olaparib; HPLC purity 99.81%, yield 82.3 %.
Embodiment 3
[0045]
[0046] At 10°C, add 2000ml of N,N-dimethylformamide into the reaction kettle, and add 4-(4-fluoro-3-(piperazine-1-carbonyl)benzyl)naphthyridine-1( 2-hydrogen)-ketone (40g, 0.109mol), HOBT (17.67g, 0.131mol), cyclopropanecarboxylic acid (12g, 0.139mol) g, finally added triethylamine (19.66g, 0.194mol, 27mL), 20 Stir at ℃ for about 7 hours. After the reaction, add 800ml of water, stir, and slowly precipitate solids, suction filter, wash, and dry to obtain 43.2 g of white solids, namely olaparib; HPLC purity 99.92%, yield 91.2% .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com