Resolution method of cyclic aralkyl surface chiral compound
A technology for chiral compounds and cycloalkanes, which is applied in the field of asymmetric catalytic synthesis, can solve problems such as the limited scope of substrates, and achieve the effects of high reactivity and enantioselectivity, mild reaction conditions, and easy availability of raw materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-7
[0035] Optimization of conditions
[0036] Change the chiral phosphine nitrogen ligand, the type of organic solvent and the amount of boric acid compounds.
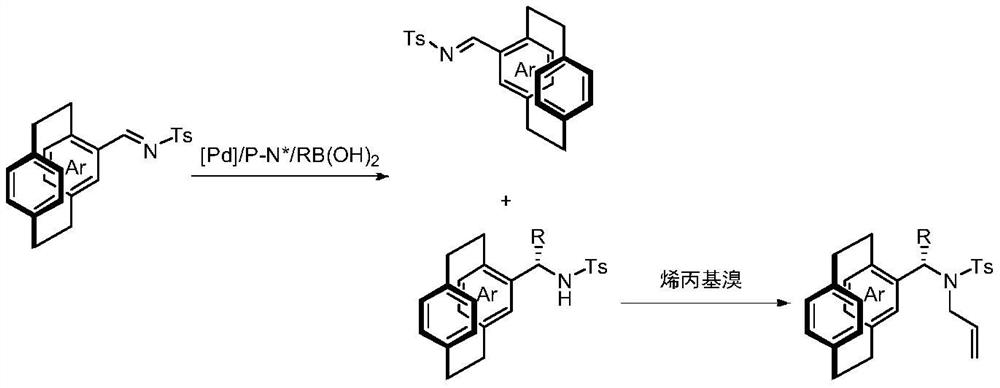
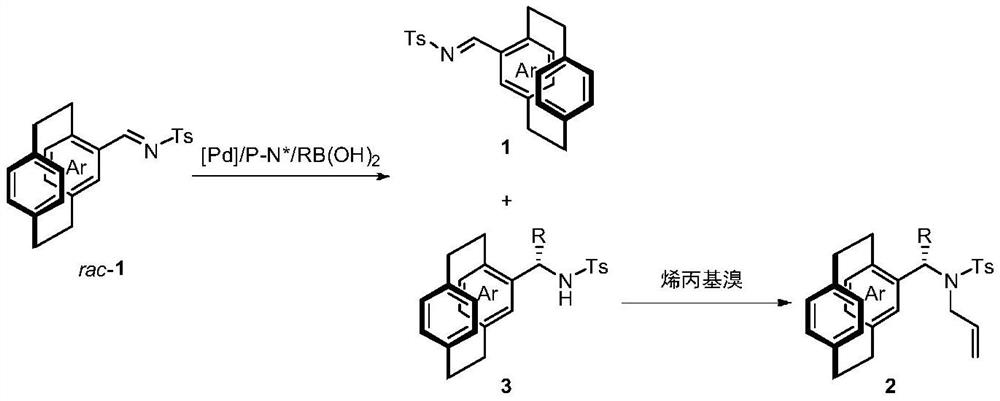
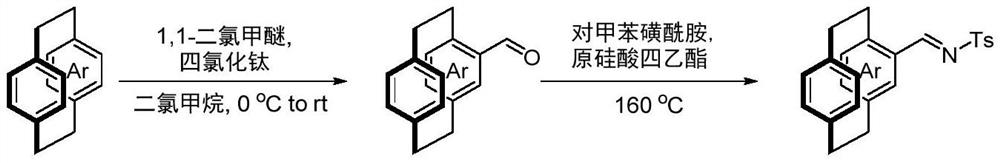
[0037] Put palladium trifluoroacetate (5 mol% of the amount of 1a) and chiral phosphine nitrogen ligand (5 mol% of the amount of 1a) into the reaction bottle, add 1 ml of acetone after nitrogen replacement, and stir at room temperature for 1 h. Concentrate in vacuo then, add 2 milliliters of organic solvents under nitrogen, ring aralkylimine 1a (0.1 mmol) and phenylboronic acid (150 mol% of 1a consumption in embodiment 1-6, be 100mol of 1a consumption in embodiment 7 %). React at 60°C for 15h. After removing the solvent, the pure hichiral cycloarylalkylimine 1a and the product precursor 3a were separated by direct column chromatography. Dissolve the product precursor 3a in 1ml of N,N-dimethylformamide in a reaction flask, add 6mg of sodium hydride (60% mass fraction) at 0°C, then add 26μl of allyl bromide, and stir at ...
Embodiment 8-19
[0042] Resolution of cycloarylalkylimine compounds with a series of boronic acids.
[0043] Put palladium trifluoroacetate (5 mol% of 1 dosage) and chiral phosphine nitrogen ligand L2 (5 mol% of 1 dosage) into the reaction bottle, add 1 ml of acetone after nitrogen replacement, and stir at room temperature for 1 h. Then concentrate in vacuo, add 4 milliliters of trifluoroethanol under nitrogen, ring aralkylimine 1 (0.2 mmol, ring aralkylimine used in embodiment 8-17 is rac-1a, ring aralkylimine used in embodiment 18-19 Alkylimine is rac-1b) and arylboronic acid (100mol% of 1 dosage), react at 60°C for 15h. After removing the solvent, the pure hichiral cycloarylalkylimine 1 and the product precursor 3 were separated by direct column chromatography. Dissolve the product precursor 3 with 2ml N,N-dimethylformamide in the reaction flask, add 12mg sodium hydride (60% mass fraction) at 0°C, then add 52μl allyl bromide, and stir at room temperature for 2h The product 2 was separated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com