Copolymer and method for producing polyurethane
A technology of copolymerization and copolymerization reaction, which is applied in the field of preparation of copolymers, can solve problems such as the treatment of a large amount of waste acid, and achieve the effect of a simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] [Synthesis of THF-polycaprolactone copolymer]
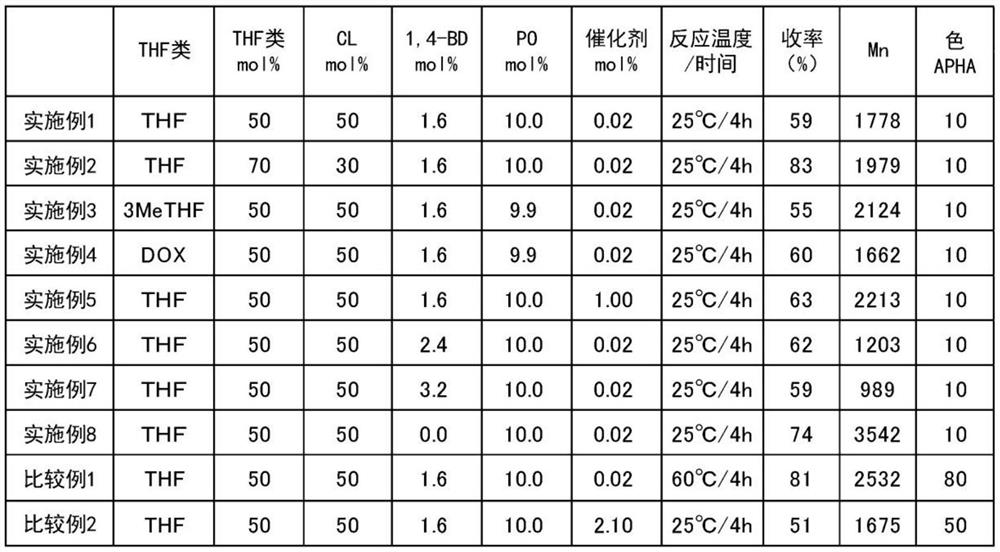
[0061] Add 63.3 g (0.56 mol) of ε-caprolactone (B) to a 500 mL four-neck separable flask (50 mol % relative to the total number of moles of five-membered cyclic ether (A) and lactones (B) , manufactured by Tokyo Chemical Industry Co., Ltd.), THF (A) 40.0 g (0.56 mol, 50 mol % relative to the total number of moles of the five-membered cyclic ether (A) and lactones (B)), 1,4 -Butanediol (C) 1.7g (0.02 mol, 1.6 mol% relative to the total number of moles of the five-membered cyclic ether (A) and lactones (B), manufactured by Kishida Chemical Co., Ltd.), tungsten phospho Acid (catalyst) 0.6g (0.0003 mol, 0.02 mol% relative to the total number of moles of the five-membered ring cyclic ether (A) and lactones (B), manufactured by Nippon Shinkoku Co., Ltd.), and a thermometer, nitrogen gas Seals, stirring devices. After stirring at 25° C. for 15 minutes, 6.4 g (0.11 mol) of propylene oxide (D) was added thereto (10.0 mol % relati...
Embodiment 2
[0063] [Synthesis of THF-polycaprolactone copolymer]
[0064] Polymerization was carried out under the same conditions and operations as in Example 1, except that ε-caprolactone in Example 1 was changed to 37.7 g (0.33 mol) and THF was changed to 55.5 g (0.77 mol). Table 1 shows the combination ratio of each raw material, the reaction temperature / stirring time, and the yield, number average molecular weight (Mn) and color number of the obtained polymer.
Embodiment 3
[0066] [Synthesis of 3-methyl-THF-polycaprolactone copolymer]
[0067] Polymerization was carried out under the same conditions and operations as in Example 1, except that 40.0 g (0.56 mol) of THF in Example 1 was replaced with 48.2 g (0.56 mol) of 3-methyl-THF. Table 1 shows the combination ratio of each raw material, the reaction temperature / stirring time, and the yield, number average molecular weight (Mn) and color number of the obtained polymer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com