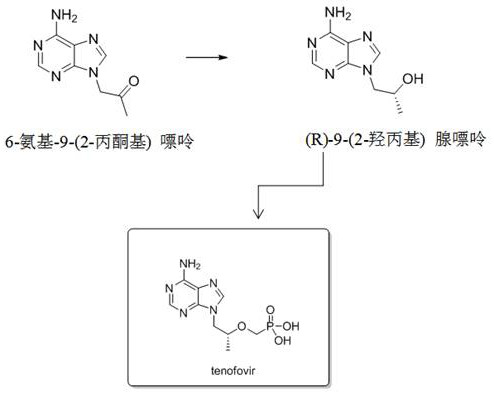
Application of dehydrogenase to preparation of (R)-9-(2-hydroxypropyl) adenine
A technology of dehydrogenase and adenine, applied in the field of genetic engineering and enzyme engineering, can solve the problems of unstable catalytic rate, unstable salt-forming catalytic rate, etc., and achieve the effect of efficient and economical production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
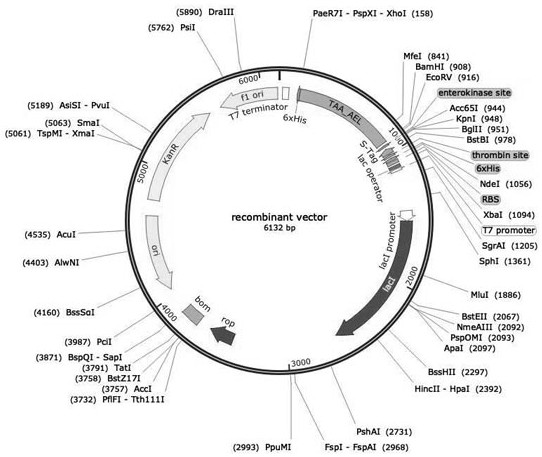
[0028] Example 1 Construction of Dehydrogenase Recombinant Strain
[0029] Design the upstream and downstream primers F and R of the dehydrogenase gene (TAA_AEL) (as shown in Table 1), use the pUC57-TAA_AEL constructed by whole gene synthesis as a template, and amplify the gene containing XhoI and BamHI restriction sites by PCR. TAA_AEL. The PCR conditions were: 98°C for 3 min, 98°C for 30 s, 55°C for 90 s, 72°C for 90 s, 30 cycles. PCR amplification system: template 1.5 μL, upstream and downstream primers 1.5 μL each, sterilized double distilled water 20.5 μL, PrimerSTAR Mix 25 μL. The gel recovery kit was used to purify and recover the PCR product, and the concentration of the recovered product was checked by electrophoresis. XhoI, BamHI enzyme-digested gel recovery product (target gene TAA) and pET-30a plasmid (expression vector), gel recovery kit to purify and recover the enzyme-digested gel recovery product, gel recovery kit to digest the plasmid Purify and recover, an...
Embodiment 2
[0034] Example 2 Fermentative production of (R)-9-(2-hydroxypropyl)adenine by recombinant bacteria
[0035] (1) Preparation of dehydrogenase crude enzyme solution
[0036] The recombinant bacteria constructed in Example 1 were inoculated in 5 mL of LB medium, cultured overnight at 37 °C with shaking, and transferred to LB medium the next day according to the inoculum size of 1%, and cultured at 37 °C until OD600=0.6, adding 50 μl of 0.5 mol / L IPTG was induced at 18°C for 14 h, and the bacterial cells were collected by centrifugation, washed with normal saline, and crushed to obtain the crude dehydrogenase enzyme solution.
[0037] (2) Fermentative production of (R)-9-(2-hydroxypropyl)adenine by recombinant bacteria:
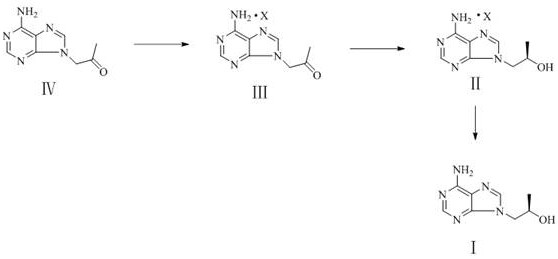
[0038] Add 50 mL of dehydrogenase crude enzyme solution obtained by crushing wet cells containing 5 g of dehydrogenase and 10 g of substrate compound II into a 250 mL Erlenmeyer flask, and shake well. Subsequently, 300 μL of 100 mg / mL NADP+coenzyme mother sol...
Embodiment 3
[0041] Example 3 Fermentative production of (R)-9-(2-hydroxypropyl)adenine by recombinant bacteria
[0042] Add 50 mL of the dehydrogenase crude enzyme solution obtained by crushing wet cells containing 5 g of dehydrogenase prepared in step (1) of Example 2 and 10 g of substrate compound II into a 250 mL Erlenmeyer flask, and shake well. Subsequently, 300 μL of 100 mg / mL NADP+coenzyme mother solution (final concentration of the reaction system 0.2 mg / mL) and 5 mL of IPA (isopropanol) 5 g of isopropanol dehydrogenase enzyme powder were added. Finally, dilute the reaction system to 100 mL with 0.2M pH 6.0 PB buffer and shake well. The 100 mL reaction system was placed on a shaker at 35 °C and 220 rpm for reaction.
[0043]
[0044] Monitor the reaction process and add IPA. Add 1mL IPA every 6 hours. At the same time, take a sample to monitor the reaction. Take 10 μL of the reaction solution, add 200 μL of ethyl acetate to extract, centrifuge, and take the supernatant for TLC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com