Cyclosporin ophthalmic emulsion and preparation method thereof
A technology for cyclosporine and ophthalmic use, which is applied in the field of cyclosporine ophthalmic emulsion and its preparation, which can solve the problems of prolonging drug release time and inapplicability, and achieve the effect of strengthening effect, promoting healing and improving absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
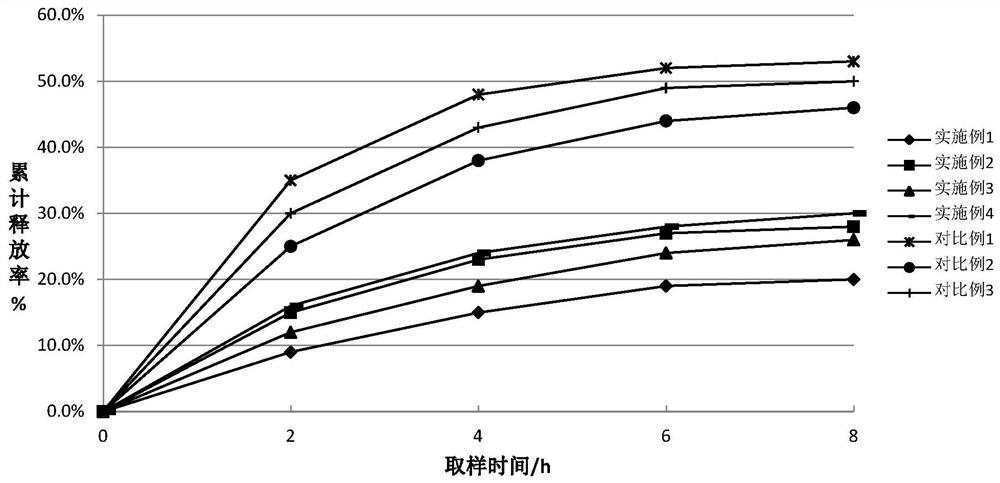
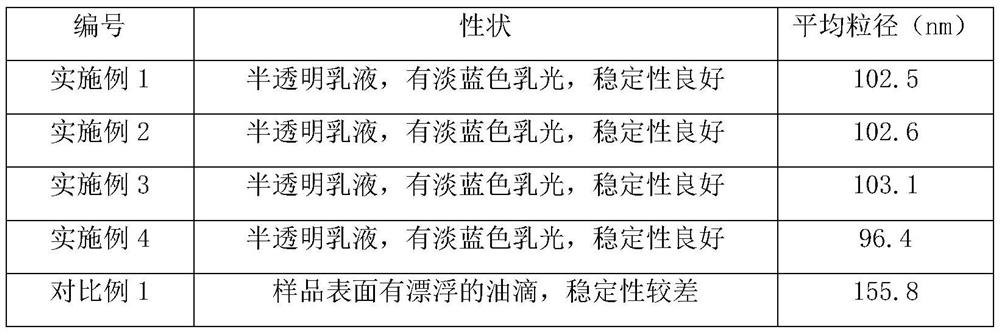
Examples
Embodiment 1
[0026] The cyclosporine ophthalmic emulsion prepared in the present embodiment, by mass percentage: cyclosporine 0.03%, collagen 2.0%, castor oil 1.4%, glycerin 2.2%, anthocyanin 0.06%, polysorbate 80 0.8%, remaining The amount is double distilled water, and the anthocyanins are ginkgo anthocyanins with a purity of 90%.
[0027] Above-mentioned cyclosporine ophthalmic emulsion preparation method:
[0028] S1. Under nitrogen protection, weigh castor oil, polysorbate 80, and cyclosporine, mix and stir at 70°C for 10 minutes to obtain the mixed solution I;
[0029] S2. Under nitrogen protection, add 80% by weight of double-distilled water, glycerin, and collagen to the mixed solution I, and shear and emulsify at 10,000 rpm at 70°C for 45 minutes to obtain the mixed solution II;
[0030] S3. Dissolve the anthocyanins in the remaining double-distilled water and mix with the mixed solution II, disperse at 60°C and 5500rpm for 100min, adjust the pH to 6.5-8.0, stir for 40min, and fi...
Embodiment 2
[0032] The cyclosporine ophthalmic emulsion prepared in the present embodiment, by mass percentage: cyclosporine 0.05%, collagen 2.5%, castor oil 1.2%, glycerin 2.0%, anthocyanin 0.05%, polysorbate 80 0.9%, remaining The amount is double distilled water, and the anthocyanins are ginkgo anthocyanins with a purity of 90%.
[0033] The preparation method of the cyclosporine ophthalmic emulsion of this embodiment is the same as that of Example 1.
Embodiment 3
[0035] The cyclosporine ophthalmic emulsion prepared in the present embodiment, by mass percentage: cyclosporine 0.05%, collagen 2.5%, castor oil 1.2%, glycerin 2.0%, anthocyanin 0.08%, polysorbate 80 0.9%, remaining The amount is double distilled water, and the anthocyanins are ginkgo anthocyanins with a purity of 88.5%.
[0036] The preparation method of the cyclosporine ophthalmic emulsion of this embodiment is the same as that of Embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com