Plant-derived flavonoid antibacterial compound and application thereof
A technology of flavonoids and compounds, applied in antibacterial drugs, medical preparations containing active ingredients, organic chemistry, etc., can solve problems such as neglect of chemical diversity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1, the screening of the flavonoid antimicrobial compound of plant source
[0027] It is estimated that there are about 450,000 species in the plant kingdom, and 31,142 species of vascular plants in 301 families, 3,408 genera, and 31,142 species of vascular plants in China are recorded in Flora of China. Flavonoids are widely distributed in plants and perform many functions. Currently, more than 5,000 natural flavonoids have been identified from various plants. The present invention first searched 85 flavonoids from the literature, including 23 flavones, 20 isoflavones, 13 flavanones, 10 chalcones and 19 ketone. Structurally, it mainly contains isopentenyl groups. According to statistics, these compounds come from 39 families and 271 different plants. According to the CLSI2019 standard guidelines (executive standards for antimicrobial susceptibility testing), its antibacterial activity against bacterial pathogens was determined, and the antibacterial effec...
Embodiment 2
[0045] Embodiment 2, the screening of the flavonoid antimicrobial compound of plant source
[0046] In most flavonoids, prenylation can be detected. It has been reported in the literature that flavonoids exert antibacterial activity, which is related to isopentenyl. In this study, through the comparison of structure-activity relationship, we found that the prenylated group is an essential group for flavonoids to exert antibacterial activity, but not all prenylated flavonoids have antibacterial activity. Of the 85 compounds, 79 compounds had a prenyl group, of which 46 compounds had one prenyl group, 25 had two prenyl groups, and 8 had three prenyl groups base. However, of the 85 compounds, 37 compounds had antimicrobial activity against MRSA, 36 of which were prenylated and 1 compound was not prenylated. Among them, two prenylated (69.2%, 18 / 26) flavonoid antimicrobial compounds were dominant compared with one or three prenylated compounds. And, the isopentenyl is located ...
Embodiment 3
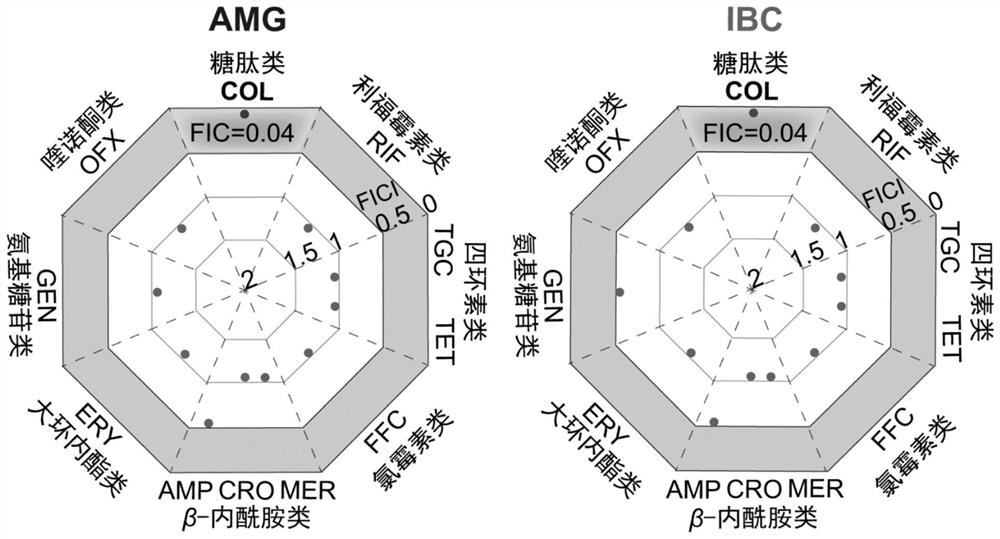
[0047] Embodiment 3, flavonoid compound AMG, the mensuration of IBC antibacterial activity
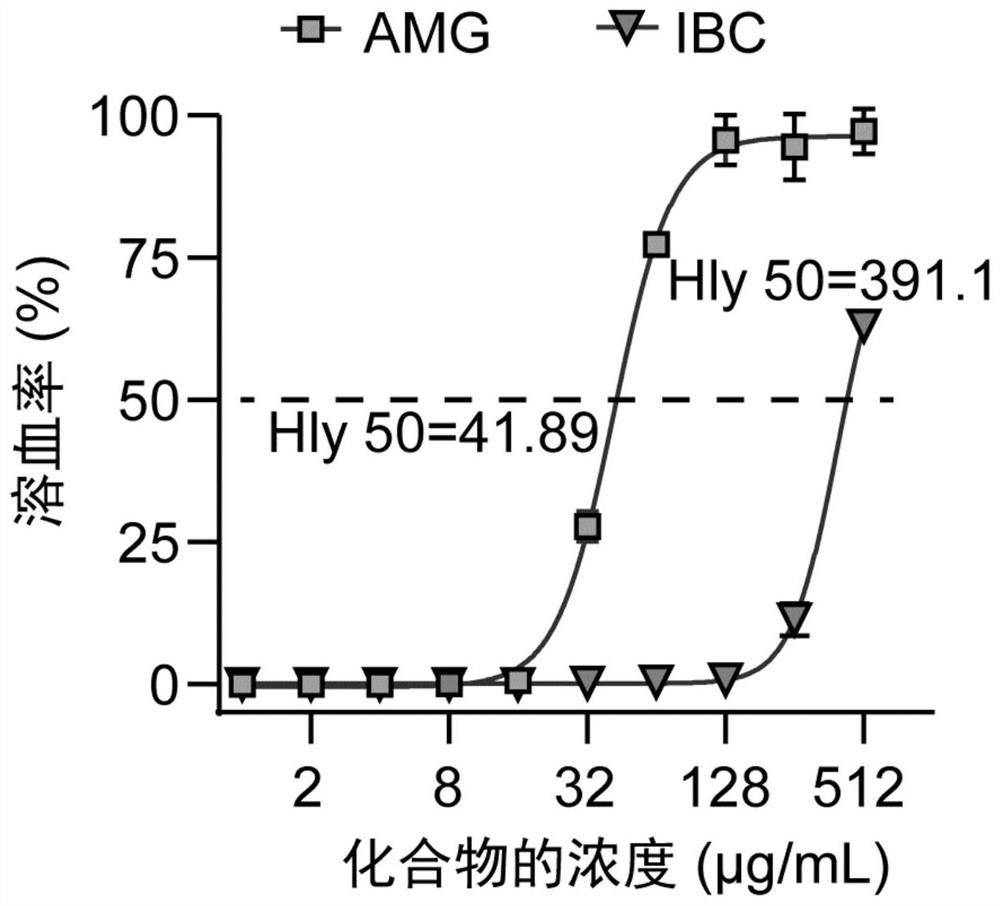
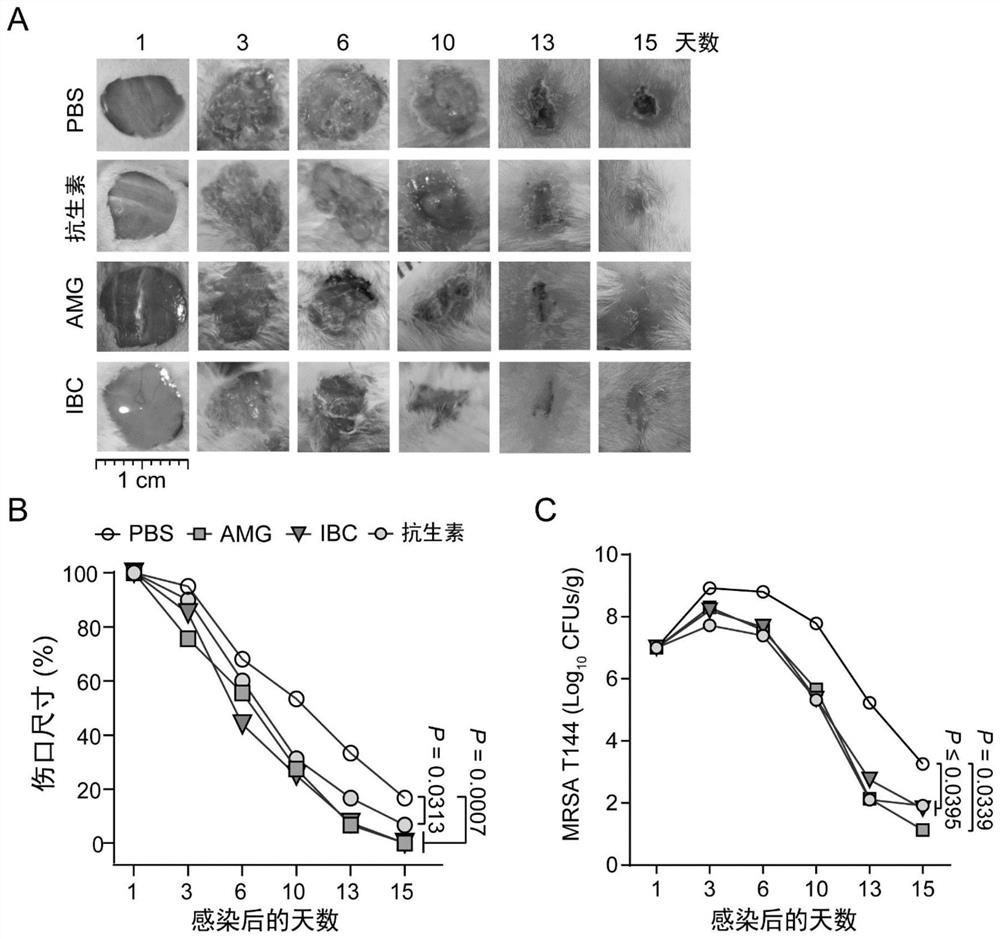
[0048] In order to better discover the leading compound with the best effect, this study screened out phlavin, 5,7,4'-trihydroxy-6,3'-diprenyl isoflavone, light Glycyrrhizol, Isopsoralen Chalcone (IBC) and α-Mangostin (AMG). Since it was found that AMG and IBC not only have better activity against Gram-positive bacteria and synergistic Colistin activity against Gram-negative bacteria, but also have lower hemolytic and cytotoxicity, so AMG and IBC have made further progress antibacterial activity assay.
[0049] In this part, the antibacterial activities of AMG and IBC against MDR bacteria MRSA (23 strains) and VRE (54 strains) were mainly determined. It also includes quality control strains ATCC 29213, ATCC 25922 and control strain E.coli B2.
[0050] See Table 2 for the strains to be tested.
[0051] Table 2
[0052]
[0053]
[0054]
[0055]
[0056] Note: a. Staphy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com