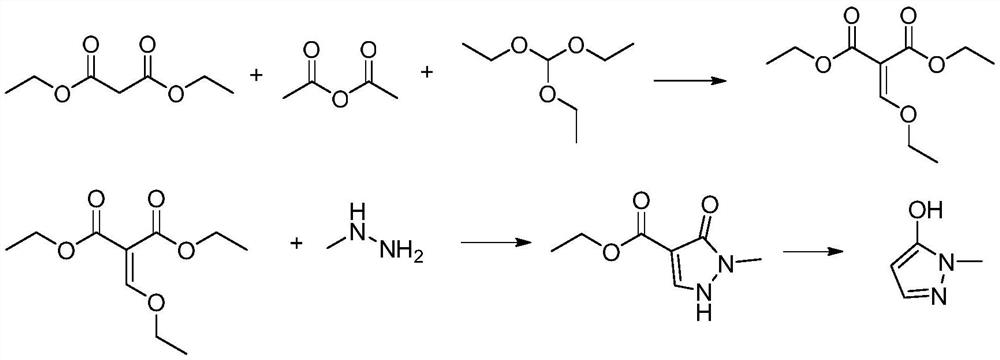
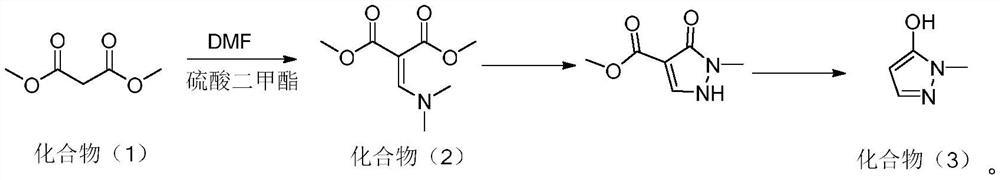
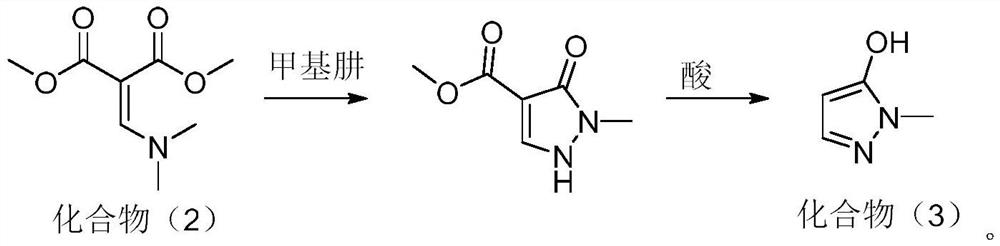
Preparation method of pyrazole type herbicide intermediate 1-methyl-5-hydroxypyrazole
A technology of hydroxypyrazole and methyl, which is applied in the field of preparation of pyrazole herbicide intermediates, can solve the problems of unfavorable large-scale production, lower yield, poor selectivity, etc., and achieve easy recovery, low price, high yield and purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] This embodiment provides a method for preparing a pyrazole herbicide intermediate 1-methyl-5-hydroxypyrazole, comprising:
[0037] (1) In a 250ml four-neck flask equipped with a mechanical stirrer, a thermometer, and a condenser tube, add 50g of solvent toluene, 16.1g of DMF (N,N-dimethylformamide) and 27.8g of alkylating agent dimethyl sulfate Add 26.4g (0.2mol) of dimethyl malonate dropwise at 70°C with stirring, drop the temperature to 50°C and add 30.24g (0.24mol) of N,N-diisopropylethylamine dropwise, the dropwise addition is completed within 2 hours ; Insulated at 60°C for 5 hours, and post-processed to obtain compound (2);
[0038] (2) Cool the toluene solution containing compound (2) to 0° C., add 21.9 g of methylhydrazine (content 42%, 0.2 mol), and keep warm for 4 hours to carry out cyclization reaction;
[0039] After treatment, add 56g of sulfuric acid (content 70%, 0.4mol) and heat up to 70°C to react for 8 hours, add alkali to neutralize and remove toluen...
Embodiment 2
[0041] This embodiment provides a method for preparing a pyrazole herbicide intermediate 1-methyl-5-hydroxypyrazole, comprising:
[0042] (1) In a 250ml four-necked flask equipped with a mechanical stirrer, a thermometer, and a condenser, add 60g of dichloroethane as a solvent, 16.1g of DMF and 27.8g of dimethyl sulfate, stir and raise the temperature at 70°C and add dimalonate di 26.4g (0.2mol) of methyl ester, drop the temperature to 50°C and add 24.24g (0.24mol) of triethylamine dropwise, the dropwise addition is completed within 2h, keep warm at 50°C for 5h to obtain compound (2);
[0043] (2) Cool the reaction solution containing compound (2) to 0°C and add 21.9 g of methylhydrazine (content 42%, 0.2 mol) for 4 hours of reaction;
[0044] After treatment, add 56g of sulfuric acid (content 70%, 0.4mol) and heat up to 65°C for 8 hours, add alkali to neutralize, remove the solvent, add 40g of ethanol for beating and filter, and the filtrate is precipitated to obtain 18.6g of...
Embodiment 3
[0046] This embodiment provides a method for preparing a pyrazole herbicide intermediate 1-methyl-5-hydroxypyrazole, comprising:
[0047] (1) In a 250ml four-necked bottle equipped with mechanical stirring, a thermometer, and a condenser, add 60g of dichloroethane, 16.1g of DMF and 27.8g of dimethyl sulfate, stir and raise the temperature at 70°C, and add dimethyl malonate dropwise 26.4g (0.2mol) of ester, cooled to 50°C and added dropwise 24.24g (0.24mol) of triethylamine, the dropwise addition was completed within 2h, kept at 60°C for 5h and treated to obtain compound (2);
[0048](2) cooling the ethylene dichloride solution containing compound (2) to -5°C, adding hydrazine hydrate 12.5g (content 80%, 0.2mol), adding methylating reagent dimethyl sulfate 27.72g ( 0.22mol), insulation reaction 3 hours after adding;
[0049] Add 56g of sulfuric acid (content 70%, 0.4mol) and heat up to 70°C for 6 hours, add alkali to neutralize and remove the solvent, add 40g of ethanol to bea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com