Chiral purification method of compound C
A purification method and compound technology, applied in organic chemistry methods, organic chemistry, etc., can solve the problems of cumbersome operation, low ee value, high production cost, etc., and achieve the effects of high yield, less three wastes, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
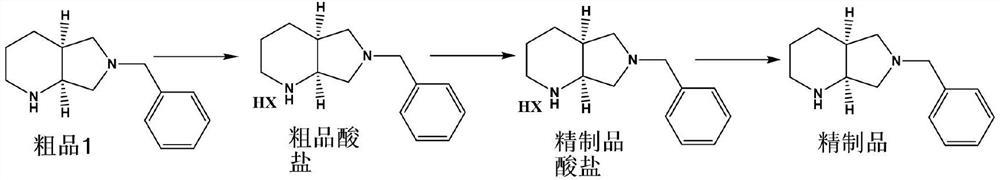
[0033] A method for purifying (S,S)-8-benzyl-2,8-diazabicyclo[4.3.0]nonane with a chiral purity ee value of 90.2%, comprising the following steps:
[0034] (1) Preparation of acid salt: 20.0 g of crude product 1 with an ee value of 90.2%, namely (S,S)-8-benzyl-2,8-diazabicyclo[4.3.0]nonane, 100 mL of ethanol, Add 31% hydrochloric acid solution to control pH 1-2, then distill off ethanol under reduced pressure to obtain (S,S)-8-benzyl-2,8-diazabicyclo[4.3.0]nonane hydrochloride 23.4 g. Then add 120mL of anhydrous methanol, heat up to 80°C for 1 hour, slowly lower to room temperature 20-30°C for 1 hour to crystallize, filter and wash with absolute ethanol, and dry the solid to obtain 20.2g of refined acid salt. 86.4%, ee value 99.6%;
[0035] (2) Stripping alkaloids: Add 50mL purified water, 3.2g caustic soda and 50mL*2 dichloromethane to the refined acid salt obtained in step (1) for extraction, dry over anhydrous sodium sulfate and distill off dichloromethane 17 g of (S,S)-...
Embodiment 2
[0037] A method for purifying (S,S)-8-benzyl-2,8-diazabicyclo[4.3.0]nonane with a chiral purity ee value of 95.2%, comprising the following steps:
[0038](1) Preparation of acid salt: 20.0 g of crude product 1 with an ee value of 95.2%, namely (S,S)-8-benzyl-2,8-diazabicyclo[4.3.0]nonane, 100 mL of ethanol, Add 31% hydrochloric acid solution to control pH 1-2, then distill ethanol off under reduced pressure to obtain (S,S)-8-benzyl-2,8-diazabicyclo[4.3.0]nonane hydrochloride 23.4g. Then add 120mL of absolute ethanol, raise the temperature to 60°C for 2 hours, lower the temperature to 20-30°C for 1 hour to crystallize, filter and wash with absolute ethanol, and then dry the solid to obtain 21.3g of refined acid salt with a yield of 91.3%. , ee value 99.9%;
[0039] (2) Stripping alkaloids: Add 50mL of purified water and 3.5g of caustic soda to the refined acid salt obtained in step (1), and then add 50mL*2 cyclohexane to extract, dry over anhydrous sodium sulfate, and evapor...
Embodiment 3
[0041] A method for purifying (S,S)-8-benzyl-2,8-diazabicyclo[4.3.0]nonane with a chiral purity ee value of 95.2%, comprising the following steps:
[0042] (1) Preparation of acid salt: 20.0 g of crude product 1 with an ee value of 95.2%, namely (S,S)-8-benzyl-2,8-diazabicyclo[4.3.0]nonane, 100 mL of ethanol, Add 31% hydrochloric acid solution to control pH 1-2, then distill ethanol off under reduced pressure to obtain (S,S)-8-benzyl-2,8-diazabicyclo[4.3.0]nonane hydrochloride 23.4g. Then add 120mL mixed solvent (acetone / ethanol=9), raise the temperature to 70°C and keep it for 1 hour, lower it to room temperature at 20-30°C and keep it for 1 hour to crystallize, filter and wash with absolute ethanol, and then dry the solid to obtain refined acid salt 21.8 g, yield 93.4%, ee value 99.9%;
[0043] (2) Stripping alkaloids: Add 50mL purified water, 3.5g caustic soda and 50mL*2 toluene to the refined acid salt obtained in step (1) for extraction, then dry with anhydrous sodium s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com