Novel amine functionalized polymers and methods of preparation
一种聚合物、胺官能的技术,应用在新型胺官能化聚合物领域,能够解决限制可用性、失活等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
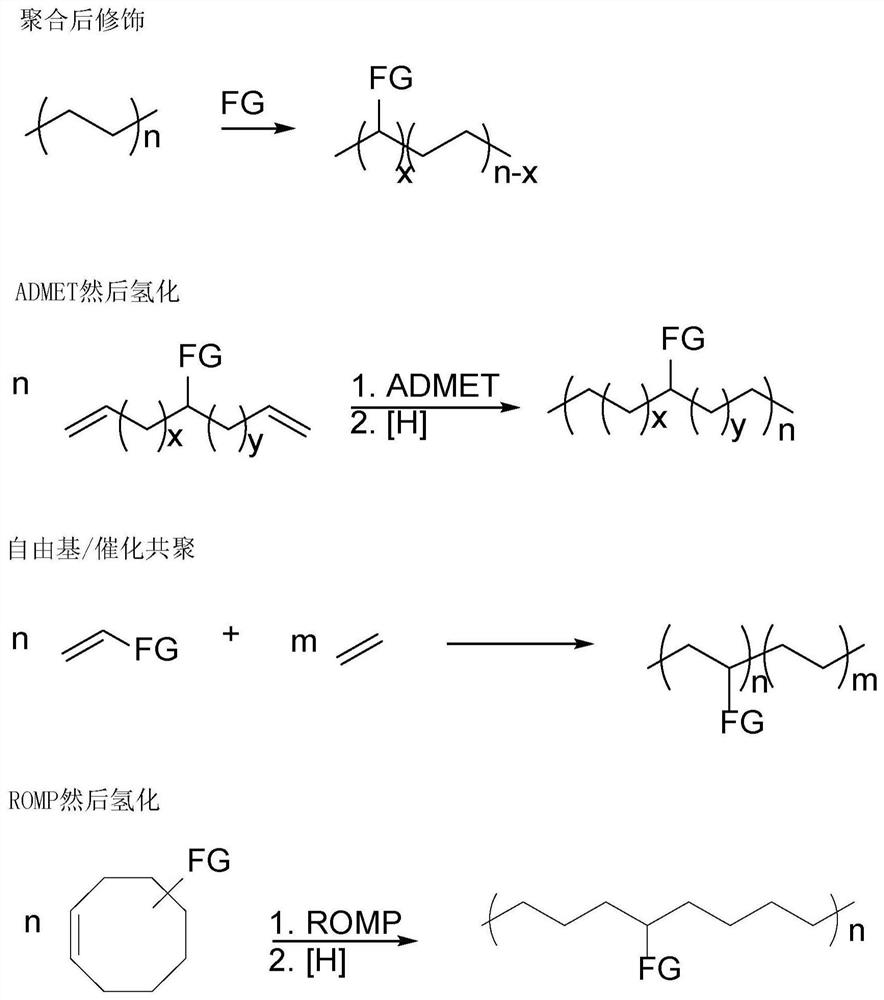
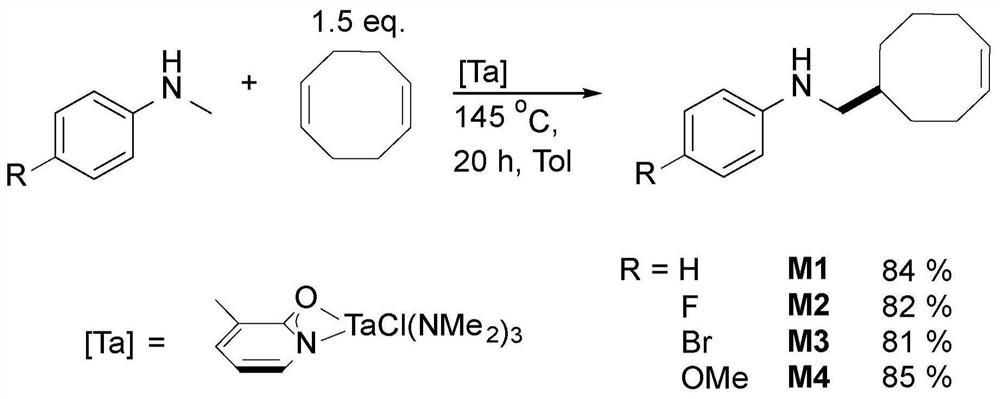
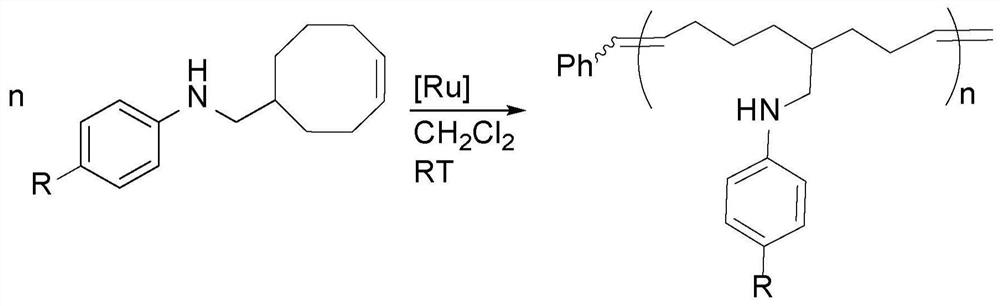
[0271] The novel amine-functionalized polymers disclosed herein were made possible by a currently developed and published catalytic synthesis using hydroaminoalkylation and ring-opening metathesis polymerization (ROMP) using Grubbs second-generation catalysts ("G2") ). This preparation method minimizes waste generation by converting commercially available starting materials into a rich variety of novel polymers without the use of additives or positioning / protecting groups. A series of cyclooctene derivatives containing secondary aromatic amines were developed in atom-economical and gram-scale preparation. The preparation of such amine-functionalized polyethylene analogues involves two steps, and optionally three steps. First, monomers are synthesized by catalytic hydroaminoalkylation of cyclic olefins such as cyclooctadiene, an aminoalkylation of olefins in an atom-economical manner. This approach avoids amine targeting or protecting groups (see figure 2 ). Second, these ...
Embodiment
[0322] Various alternative embodiments and examples are described herein. These embodiments and examples are illustrative and should not be construed as limiting the scope of the present invention. In particular, while tantalum was used as the representative Group 5 metal for these studies, one skilled in the art should expect that other Group 5 metals, especially niobium, would exhibit similar properties.
[0323] Materials and methods
[0324] The steps described herein are for the purpose of illustration and description only and should not be considered as limiting the spirit or scope of the present invention.
[0325] 1. General
[0326] All reactions were carried out under an inert atmosphere using a 2 and high vacuum (10 -3 mbar) Schlenk double manifold or filled with N 2 glove box. All glassware used was heated in an oven to over 160°C prior to use. Reactions were performed in 20 mL threaded scintillation vials equipped with Teflon-coated magnetic stir bars and...
Embodiment 2
[0376] Example 2: NMR Polymerization Studies
[0377] Polymerization of M1 was monitored by NMR spectroscopy. In a sealed NMR tube add Grubbs initiator, 100 equivalents of M1 and about 1 mL of deuterated chloroform. After 10 minutes, about 35% of the monomer was consumed; after 30 minutes, the reaction was more than 95% complete. Compared with other amine-functionalized monomers (which are not compatible with Grubbs initiator), the rapid conversion of M1 indicates that aryl-substituted secondary amines are prone to ROMP reaction. Notably, the signal at 19.2 ppm attributed to the protons of the benzylidene Ru=CHPh is still present in the spectrum throughout the polymerization (panel X), where this observation may indicate incomplete initiation of the catalyst, as well as propagating The rate is greater than the initiation rate of the catalyst.
[0378]To explore whether chain termination occurred at the completion of the reaction, an aliquot of about 25 equivalents of M2 was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com