Antibody cBIN1 and application thereof
An antibody, cbin1 technology, applied in the field of immunity, can solve problems such as decline, E-C decoupling of cardiac function, and decreased metastatic ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
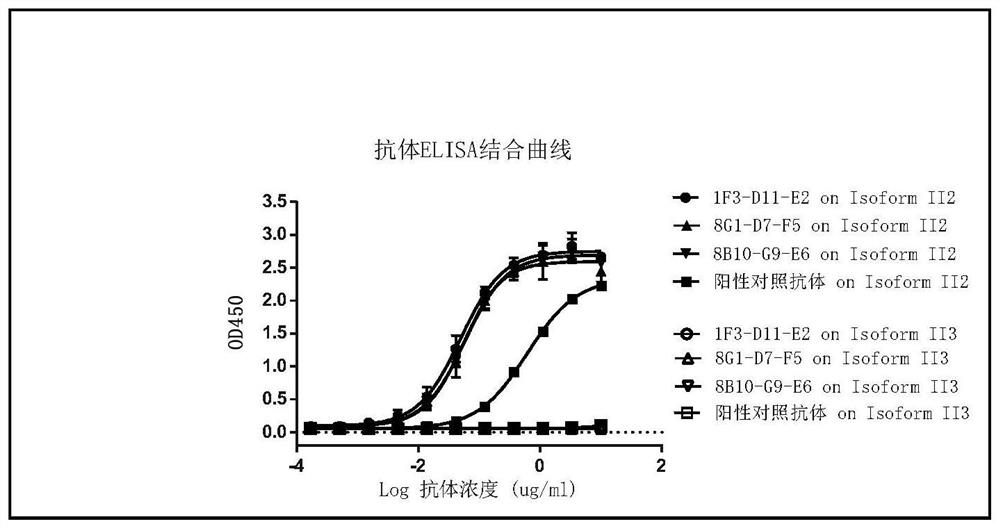
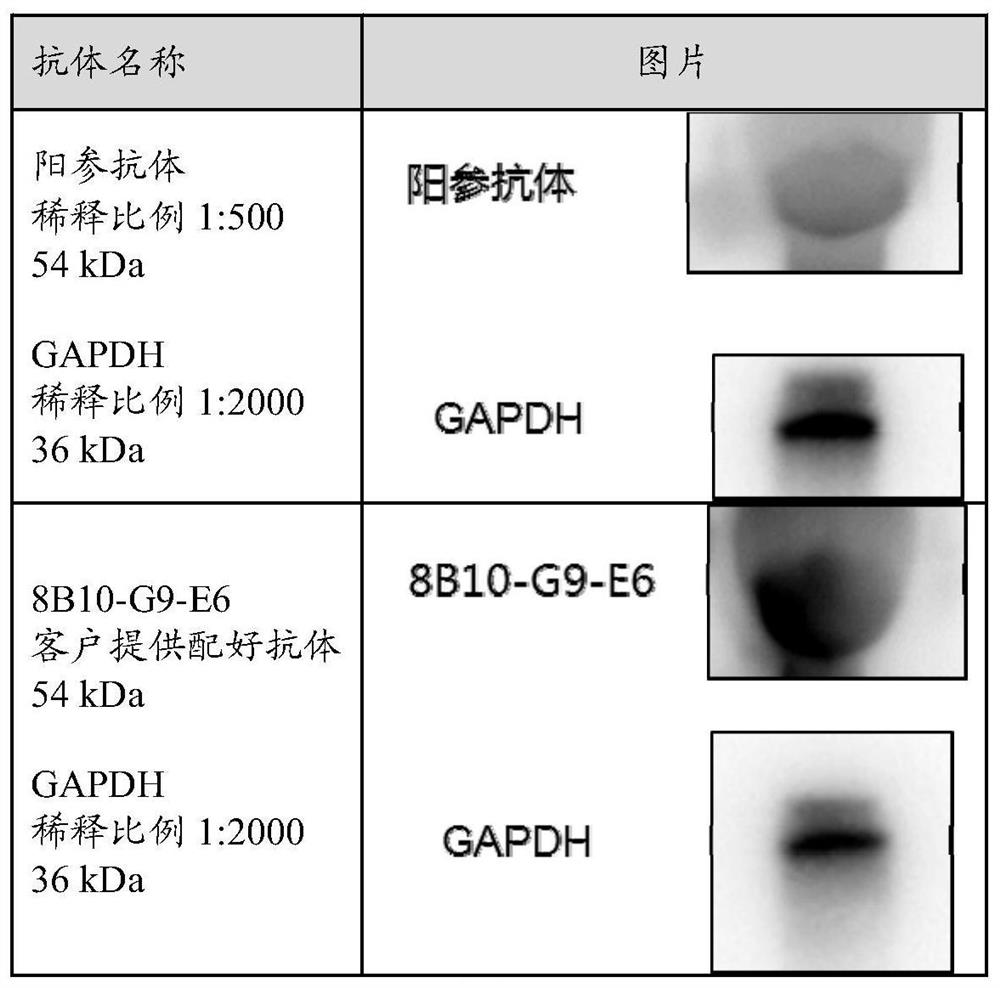
Image
Examples
Embodiment 1
[0059]Example 1: Synthesis of antigen for immunization
[0060]The antigen of the present invention is human Cardiac Bridging Integrator 1 Excmine 13 (Cbin1 EXON13) corresponding protein sequence LRKGPPPPPPPPPPPPSkevkqeqilslfedTFVPEISVTTPSQ, and the polypeptide-proof biochemical company used for immunization is synthesized by Guo Hui, in order to improve antigen-immunogenicity, the polypeptide C-terminal passed two Stearate (PEG4) coupling keylet blood blue protein (KLH, Keyhole Limpet Hemocyanin).
Embodiment 2
[0061]Example 2: Production of anti-human source Cbin1 EXON13 antibody
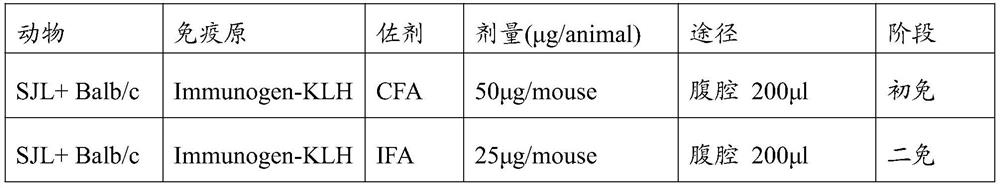
[0062]In order to obtain a mouse anti-human Cbin1 EXON13 antibody, immunization strategy (Table 1) in Table 1.1 was used to immunize different lines of mice (Balb / C, Shanghai Ling Chang; SJL, Beijing Virong Lihua). The antigen used is as described in Example 1; adjuvant includes: complete Frego adjuvant CFA (INVOGEN, INVOGEN VAC-CFA-60), IFA (INVOGEN, IQ VAC-IFA-60). After three days of enduring, the spleen cells of immunized mice were fused using polyethylene glycol cells using polyethylene glycol cells, which can express the antibody and in vitro, which can be blended in vitro, and in HAT selection Culture in the medium. The fused hybridoma cells were placed in a 96-well cell culture plate and 2 rhythmium subclon was selected by primary screening.
[0063]Table 1. Immune strategy
[0064]
[0065]
Embodiment 3
[0066]Example 3: Determination of variable region of anti-human source Cbin1 EXON13 antibody
[0067]Centrifugation collection hybridoma cells, every 5 × 106~ 10 × 106The cells were added to 1 ml of Trizol and 0.2 ml of chloroform, strenuous oscillated for 15 seconds, and 0.5 ml of isopropanol was added at room temperature, and 0.5 ml of isopropyl alcohol was added. After 10 minutes at room temperature, the ethanol was mixed and dried to give RNA. Templates RNA and primers are added to the ice bath, which makes the primer and template correctly paired the reverse transcription process, and PCR amplification. 2.5 μL of DNTP / DDNTP mixture was added to 4 microcencular tubes, and the mixture was given 37 ° C for 5 min, spare. Add 1 pmol of PCR amplified double-stranded DNA, 10Pmol sequencing bracket, 2 μl 5 × sequencing buffer, add double steamer to total volume 10 μL, 1min, 1 min, 4 ° C 10000 g from centrifugation 10S. 2 μl of pre-cooling label mixture (DCTP, DGTP, DTTP 0.75 μmol / L), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com