Preparation method of active peptide Apelin
A technology of active peptide and solid-phase synthesis method, applied in the field of biomedicine, can solve the problems of complex biotechnology and difficult large-scale production, and achieve the effect of prolonging half-life and improving activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1: The solid-phase synthesis of 2-(4-chlorophenyl)-2,2-difluoroacetic acid-QRPRLSHKGPMPF
[0018] (1) Weigh 1mmol of 2-cl-Trt resin in a solid-phase synthesizer, add 15mL of anhydrous dichloromethane (hereinafter referred to as DCM), place on a shaker and shake for 5min to fully swell the 2-Cl-Trt resin ;
[0019] (2) DCM is removed from the solid-phase synthesizer equipped with 2-Cl-Trt resin with ear washing ball;
[0020] (3) Dissolve 0.75 mmol of Fmoc-Phe in 10 mL of anhydrous DCM, add 0.75 mmol of DIPEA, then transfer to the above-mentioned solid-phase synthesizer, add 0.75 mmol of DIPEA, and react at room temperature for 1 h;
[0021] (4) Sealing: remove the reaction liquid in the solid-phase synthesizer with ear washing balls, then wash with 10 mL of anhydrous DCM, each time for 1 min, and wash 5 times in total, add the prepared volume ratio of anhydrous DCM: DIPEA: methanol =17:1:2 solution 20mL, react at room temperature for 10min;
[0022] (5) Re...
Embodiment 2
[0027] Example 2: Regulation of target active peptides on intracellular cAMP content
[0028] Using unmodified [Pyr1]-Apelin-13 as a control, the regulation of intracellular cAMP content by the target active peptide of the present invention was determined. The HEK293 cell line was transfected along with a luciferase reporter gene [cAMP response element (CRE, 4X)-luciferase] to stably express full-length human hAPLNR (amino acids 1-380 of accession number NP_005152.1). The resulting cell line HEK293 / CRE-luc / hAPLNR was cultured in DMEM containing 10% FBS, NEAA, penicillin / streptomycin and 100 μg / mL hygromycin B. HEK293 / CRE-luc / hAPLNR cells were seeded on 96-well assay plates at 20,000 cells / well in 80 μL OPTIMEM supplemented with 0.1% FBS and penicillin / streptomycin / L-glutamine and incubated at 37°C for 5 %CO 2 Incubate for 16 hours. Serial dilutions (1 :3) of unmodified [Pyrl]-Apelin-13 and the active peptide of interest were then mixed with forskolin in assay buffer (5 μΜ f...
Embodiment 3
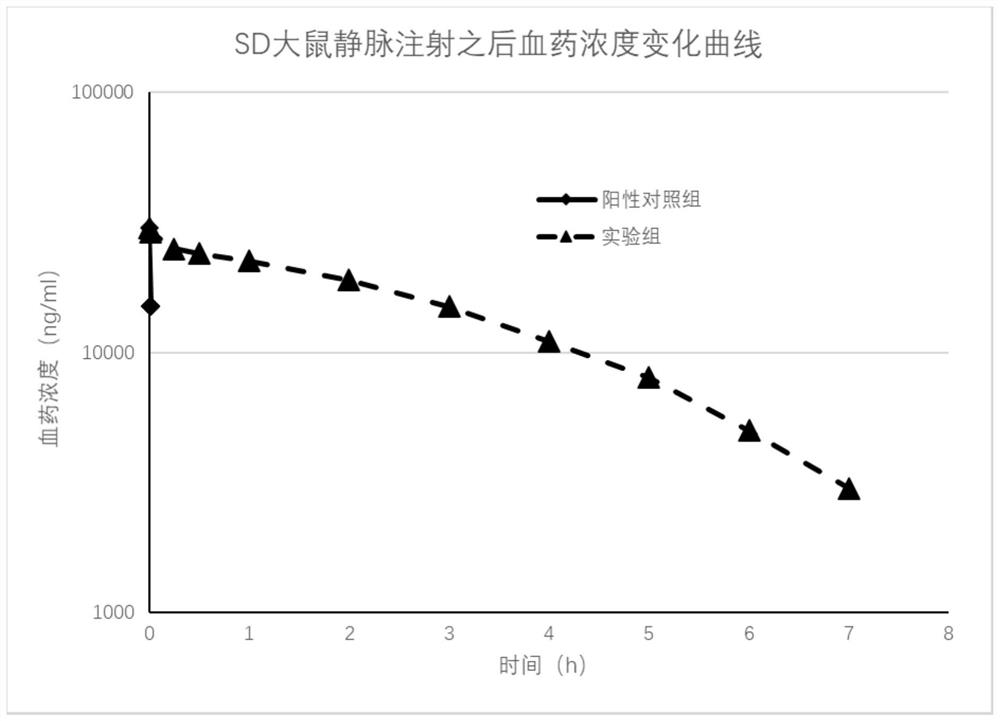
[0030] Embodiment 3: Experiment of the biological half-life of the target active peptide in rats
[0031] (1) Experimental animal information
[0032] SPF grade SD rats, 16 males, weighing 190g to 210g, were provided by the Experimental Animal Center of Wenzhou Medical University.
[0033] (2) Dosing regimen and plasma sample collection and processing
[0034] Intravenous administration, the dosage was 10mg / kg, the rats were randomly divided into positive control [Pyr1]-Apelin-13 solution group, experimental group 2-(4-chlorophenyl)-2,2-di Fluoroacetic acid-QRPRLSHKGPMPF solution group, a total of 2 groups (n=8), fixed rats, administered through the tail vein. At different time points after administration, 0.2 mL of whole blood was collected through the jugular vein cannula and placed in a 1.5 mL LEDTA-2K anticoagulant centrifuge tube. ) to seal the tube for smooth collection at the next time point. The blood sample was centrifuged at 4000g for 5 minutes at 4°C, the plasma w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com