Application of icetexane type abietane diterpene in the preparation of colorectal cancer therapeutic drug
A kind of technology of abietane diterpene and therapeutic drug, which is applied in the application field of preparation of colorectal cancer therapeutic drug, achieves strong anti-colorectal cancer activity and expands the effect of application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1 of the present invention is: the preparation method of Icetexane type abietane diterpene and its derivatives, comprising the following steps:
[0062] S1, the preparation of compound 2:
[0063]
[0064] Weigh 1.0g (3mmol) of compound 1 (carnosic acid) into a 100mL round bottom flask, add 12.5mL of toluene and 2.5mL of methanol to dissolve, slowly add 3mL of trimethylsilyldiazomethane in n-hexane dropwise at 0°C solution (2.0mol / L, 6mmol). TLC monitoring, after the reaction is complete, add 1 mL of glacial acetic acid dropwise to the reaction solution, remove the reaction solvent by rotary evaporation, add 50 mL of saturated brine, extract with ethyl acetate (3 × 30 mL), and dry the ethyl acetate layer with anhydrous sodium sulfate. Silica gel column chromatography (volume ratio of PE (petroleum ether) to EA (ethyl acrylate) was 10:1) obtained 945 mg of intermediate (yellow solid, yield 91%).
[0065] 1 H NMR (400MHz, CDCl 3 )δ7.52(s,1H),6.59(s,1H),5...
Embodiment 5
[0120] Embodiment 5 of the present invention is: flow cytometry determination (+)-Grandione influence on HCT-116 cell BiP protein translocation
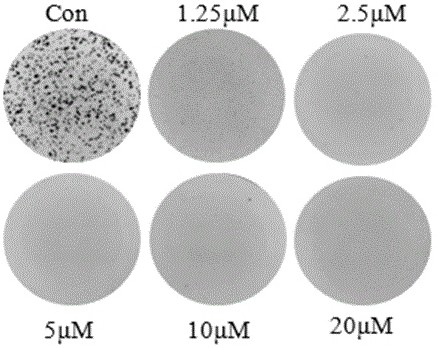
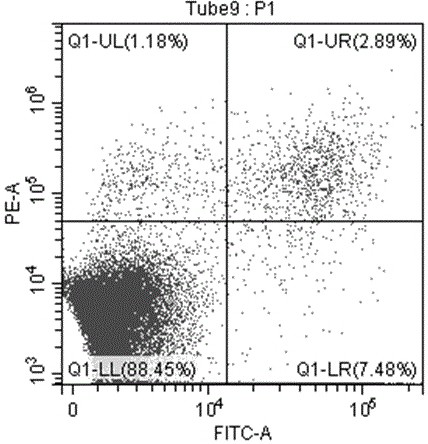
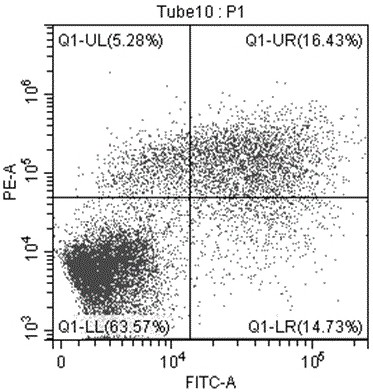
[0121] HCT116 cells in the logarithmic growth phase were digested with 0.25% trypsin and blown into single cells, 5*10 cells per well 5 Cells were seeded in 6-well plates and cultured overnight in an oven. Add 20 μM, 10 μM, 5 μM, 0 μM (blank control) (+)-Grandione respectively, and incubate for 24 hours. Cells were collected, centrifuged at 1000 rpm at 4°C for 10 min, and the supernatant was discarded. Wash the cells three times with cold PBS, add 200 μL of binding buffer working solution (1×Annexin V binding buffer) to resuspend the cells, add 10 μL of Annex in V-FITC to the cell suspension, mix gently, and react at room temperature in the dark for 15 minute. Add 300 μL of binding buffer and 5 μL of PI (propidium iodide), and detect with a flow cytometer.
Embodiment 6
[0122] Embodiment 6 of the present invention is: the influence of (+)-Grandione on HCT-116 cell BiP protein translocation is determined by immunofluorescence method
[0123] HCT116 cells in the logarithmic growth phase were digested with 0.25% trypsin and blown into single cells, 5*10 cells per well 5 Cells were seeded in a 6-well plate (including cell slides), cultured overnight in an oven to adhere to the wall, and 20 μM, 10 μM, 5 μM and 0 μM (blank control) (+)-Grandione were added and incubated for 24 hours. After washing with PBS for 3 times, fix with fixative solution (PBS solution with mass fraction of 4% paraformaldehyde) at 4°C for 12h, then wash with PBS for 3 times, and permeabilize with Triton-X100 (Triton) in PBS solution for 10min Finally, wash with PBS 3 times, 5% BSA in PBS solution blocked for 60min, add BiP antibody, wet box 4 overnight. The next day, wash 3 times with PBS, 5min each time, then add AlexaFluor 488goat anti-rabbit IgG secondary antibody, incub...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com