Injection preparation of human interleukin 10-Fc fusion protein
An interleukin and fusion protein technology, which is applied in the fields of peptide/protein components, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-8
[0049] Embodiment 1-8 of the present invention provides an injection preparation of human interleukin 10-Fc fusion protein, the preparation includes the following components:
[0050] a) a human interleukin 10-Fc fusion protein with a protein content of 2-30 mg / ml;
[0051] b) a buffer salt with a concentration of 5-100 mM;
[0052] c) 3%-10% (w / v) protein protectant;
[0053] d) an isotonic regulator at a concentration of 0-100 mM;
[0054] e) 0.005%-0.02% (w / v) nonionic surfactant;
[0055] Wherein, the pH value of the preparation is 5.0-6.0;
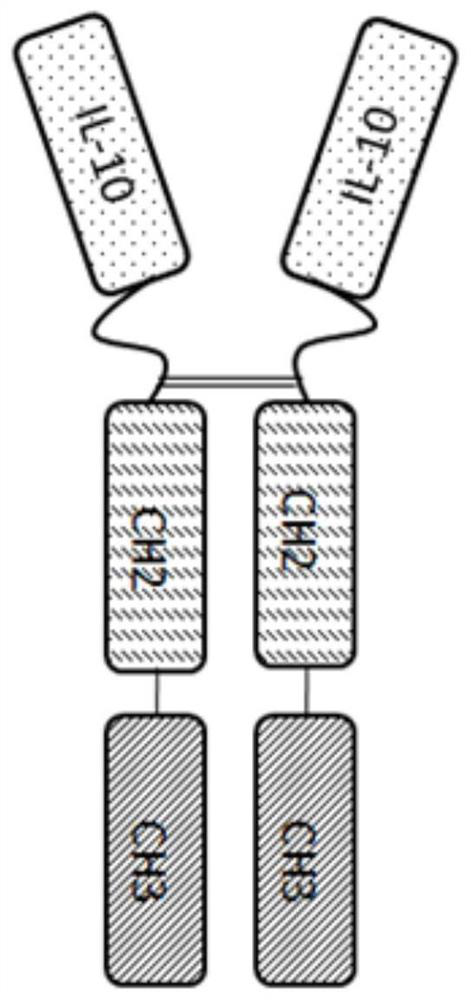
[0056] Human interleukin 10-Fc fusion protein is formed by fusing human interleukin 10 with the Fc fragment of immunoglobulin IgG through a connecting peptide; wherein, the Fc fragment is selected from one of human IgG1, IgG2 or IgG4; human leukocyte The amino acid sequence of interleukin 10 is shown in SEQ ID No:1. The general formula of the connecting peptide is [GlyGlyGlyGlyX] n ;
[0057] Wherein, X is Ser or Ala, and n is ...
Embodiment 9
[0083] Embodiment 9 of the present invention provides an injection preparation of human interleukin 10-Fc fusion protein, which preferably limits the protein content of human interleukin 10-Fc fusion protein on the basis of Examples 1-8 2-15mg / ml.
Embodiment 10
[0085] Embodiment 10 of the present invention provides an injection preparation of human interleukin 10-Fc fusion protein. Based on Examples 1-8, the injection preparation preferably limits the content of buffer salt in the preparation to 10-20 mM.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com