Nitrilase mutant with improved nitrile hydration activity specificity and application thereof
A kind of nitrilase, mutant technology, applied in the direction of hydrolase, application, enzyme, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The construction of embodiment 1 nitrilase mutant recombinant bacteria
[0029] 1. Construction of mutants
[0030] When rice nitrilase (OsNIT, GenBank accession number: AB027054, amino acid sequence SEQ ID NO.1) catalyzes the reaction of phenylacetonitrile, the ratio of amide to carboxylic acid in the product is close to 1:1. Through bioinformatics analysis, it was determined that its catalytic triplet was 196Cys-71Glu-162Lys, and then through rational design analysis, the 87th alanine, the 136th isoleucine, the 224th arginine, and the 226th Valine was subjected to site-directed saturation mutation, and a mutant with significantly increased amide content was constructed, specifically:
[0031] (1) Single point mutation: insert the rice nitrilase (OsNIT, GenBank accession number: AB027054) gene (amino acid sequence SEQ ID NO.1, nucleotide sequence is SEQ ID NO.2) into the BamH I of the pET-28b vector With the Hind III site, a recombinant plasmid was constructed. Usin...
Embodiment 2
[0052] Embodiment 2 nitrilase mutant A87M, I136Q amide content and activity determination
[0053] The reaction system consists of: 20mL Tris-HCl buffer solution (50mM, pH 8.0), 0.1g of wet thallus of nitrilase mutants A87M and I136Q prepared by the method in Example 1, methanol 0.9mL, phenylacetonitrile 100mM (phenylacetonitrile first soluble in methanol). The reaction solution was reacted at 30° C., 180 rpm for 30 minutes. Take 1 mL of sample, add 20 μL of 2M HCl to terminate the reaction, centrifuge at 12,000 rpm for 1 min, and take the supernatant. The concentration and ratio of phenylacetic acid and phenylacetamide in the product were analyzed by high performance liquid chromatography HPLC described in Example 1, and the enzyme activity was calculated. Under the same conditions, the enzyme activity of the nitrilase mutant wet cells was tested.
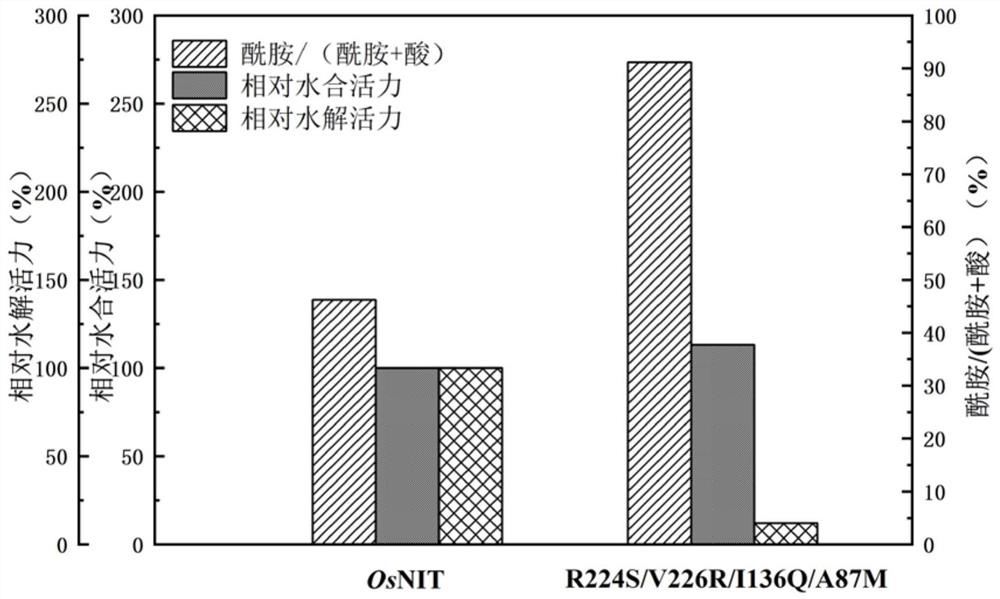
[0054] Among them, while the hydration activity of the A87M (SEQ ID NO.4) mutant was 107% of the parent, the hydrolysis activ...
Embodiment 3
[0057] Embodiment 3 Construction of nitrilase multiple mutant R224S / V226R / I136Q / A87M
[0058] Using the above method to analyze the results of single-point mutations, it was found that the mutations at the 136th and 87th positions could change the specificity of the reaction and tend to generate amides, the hydration activity was well preserved, and the hydrolysis activity was inhibited. R224S , V226R on the basis of further mutation transformation, the specific steps are as follows:
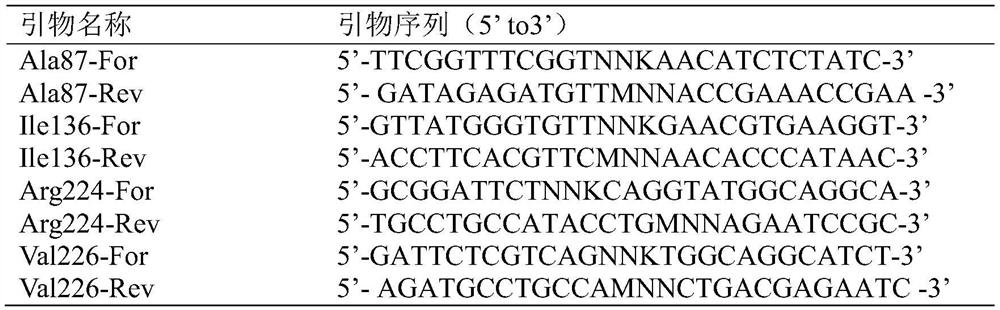
[0059] Using the R224S mutant plasmid as a template, use the V226R site-directed mutagenesis primers (Table 1 Val226-For and Val226-Rev) to carry out PCR amplification of the whole plasmid. The PCR system and amplification conditions are the same as step 1. Enzyme mutant R224S / V226R plasmid (amino acid sequence SEQ ID NO.3); then use the mutant R224S / V226R plasmid as a template, and use I136Q site-directed mutation primers (Table 1Ile136-For and Ile136-Rev) to carry out PCR amplification of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com