Preparation method of aminothiazole acetyloxime acid with controllable particle size
A technology of aminothiazole acetoxamic acid and ethyl desmethylaminothiazole acetoxamic acid, which is applied in the field of preparation of aminothiazole acetoxamic acid with controllable particle size, can solve problems such as difficult control of intermediate products, and achieve controllable particle size and simple process operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
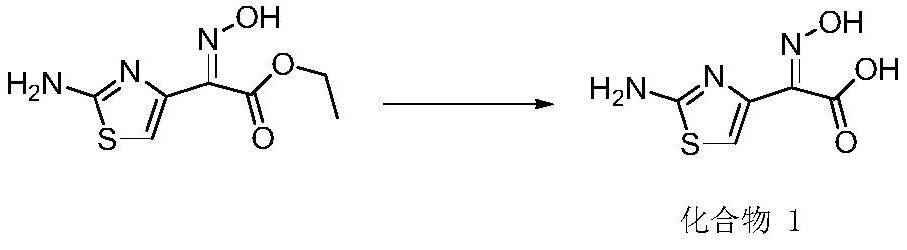
[0039] Hydrolysis process: 50.00 g of ethyl demethylaminothiaxate, 150 ml of tap water and 50 ml of methanol were placed in a 500 ml three-neck flask, and 27.00 g of sodium hydroxide was added to the system at room temperature, and stirred overnight after dropping to obtain compound 1.
[0040] Acylation process: 97.0 g of acetic anhydride was added dropwise to the system, and stirred for 4 hours after dropping to obtain compound 2.
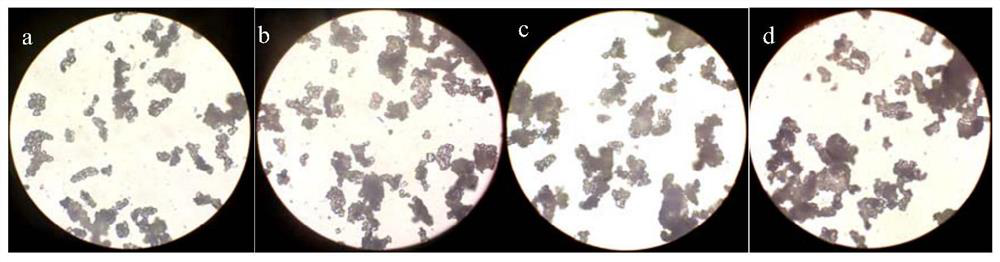
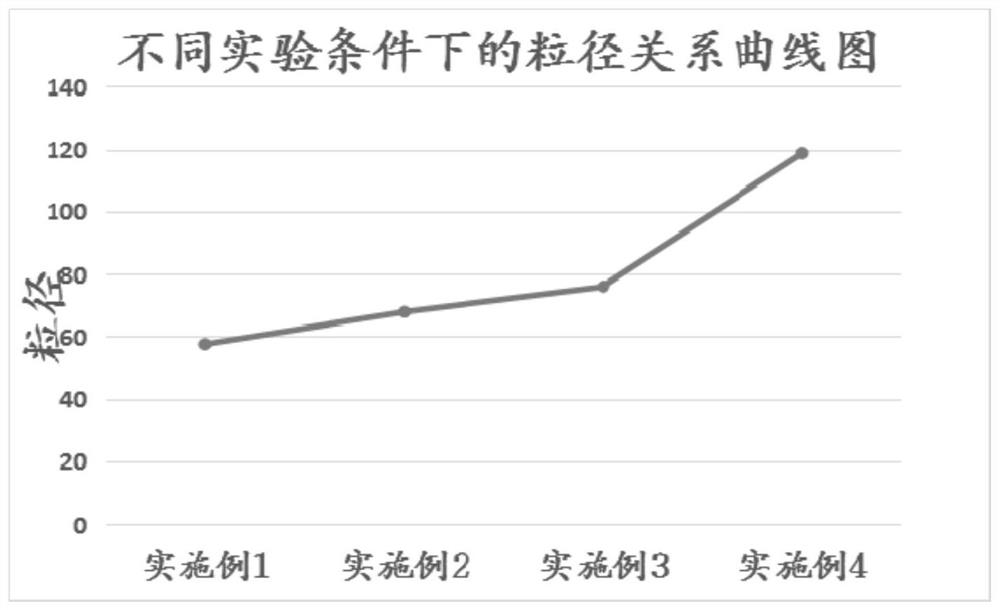
[0041] Crystallization process: Add 1100ml of tap water and 2g of activated carbon for decolorization, maintain the system temperature at 10-15°C, add 20wt.% hydrochloric acid aqueous solution dropwise to pH = 3-4, and control the dropwise addition for 45min. Suction filtration, washing with 50 ml of water three times respectively, to obtain the wet product of compound 3, and drying in vacuum to obtain 135.28 g of the dry product of compound 3, with a yield of 78.5%. Particle size detection showed that its D50% particle size was 57.559 μm.
Embodiment 2
[0043] Hydrolysis process: 50.00 g of ethyl demethylaminothiaxate, 150 ml of tap water and 50 ml of methanol were placed in a 500 ml three-necked flask, and 27.01 g of sodium hydroxide was added to the system at room temperature, and stirred overnight after dropping to obtain compound 1.
[0044] Acylation process: 97.0 g of acetic anhydride was added dropwise to the system, and stirred for 4 hours after dropping to obtain compound 2.
[0045] Crystallization process: Add 1100ml of tap water and 2g of activated carbon for decolorization, maintain the system temperature at 10-15°C, add 20wt.% hydrochloric acid aqueous solution dropwise to pH = 3-4, and control the dropwise addition for 45min. Suction filtration, washing with 50 ml of water three times respectively to obtain the wet product of Compound 3, and drying in vacuum to obtain 132.69 g of the dry product of Compound 3, with a yield of 77.0%. Particle size detection showed that its D50% particle size was 67.902 μm.
Embodiment 3
[0047] Hydrolysis process: 50.00 g of ethyl demethylthiaxamate, 150 ml of water, 30 ml of tetrahydrofuran, 192.40 g of potassium carbonate were added to the system at room temperature, and the mixture was stirred overnight to obtain compound 1.
[0048] Acylation process: 97.0 g of acetic anhydride was added dropwise to the system, and stirred for 4 hours after dropping to obtain compound 2.
[0049] Crystallization process: Add 1100ml of tap water and 2g of activated carbon for decolorization, maintain the system temperature at 20-25°C, add 30wt.% acetic acid aqueous solution dropwise to pH = 3-4, and add dropwise for 45min at a controlled time. Suction filtration and rinsing with 50ml of water three times respectively to obtain the wet product of Compound 3, which was dried in vacuum to obtain 119.42 g of the dry product of Compound 3 with a yield of 73.5%. Particle size detection showed that its D50% particle size was 76.004 μm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com