Aluminum secondary battery and aluminum storage active material thereof
A technology for aluminum secondary batteries and cathode active materials, applied in secondary batteries, battery electrodes, non-aqueous electrolyte storage batteries, etc., can solve problems such as poor cycle performance, limited reversible capacity of devices, and mismatch of high specific capacity values of metal aluminum , achieve the effects of low cost, improved electrochemical reaction kinetics, and shortened transport length
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
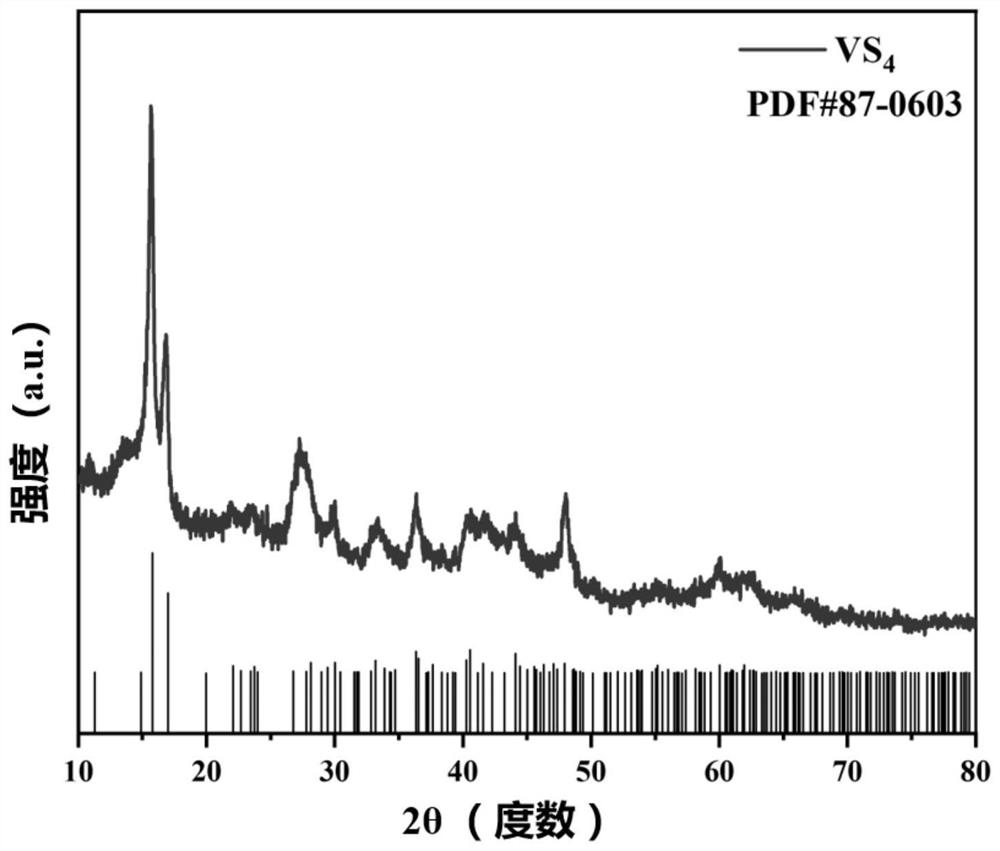
[0040] According to the molar ratio of the reaction substances being basically 1:5, 1.127 g of thioacetamide powder and 0.351 g of ammonium metavanadate powder were weighed respectively. Add 30mL deionized water to a beaker, add the weighed ammonium metavanadate, stir magnetically for 30 minutes in an oil bath at 80°C, and completely dissolve to form solution A; add 30mL ethylene glycol reagent to another beaker, add Weighed thioacetamide (CH 3 CSNH 2 ), stirred magnetically for 10 minutes to form solution B; introduced solution B into solution A, added sodium hydroxide (NaOH) to adjust the pH to 8; stirred magnetically for 30 minutes under the temperature condition of 80°C to fully mix, and transferred the above solution to 100mL In a stainless steel high-pressure reactor with a polytetrafluoroethylene liner, the solvothermal reaction was carried out at a temperature of 160°C, and the reaction time was 24 hours; after the product was naturally cooled to room temperature, it ...
preparation example 2
[0044] According to the molar ratio of the reaction substances being basically 1:5, 1.127 g of thioacetamide powder and 0.351 g of ammonium metavanadate powder were weighed respectively. Add 30mL deionized water to a beaker, add the weighed ammonium metavanadate, stir magnetically for 30 minutes in an oil bath at 80°C, and completely dissolve to form solution A; add 30mL ethylene glycol reagent to another beaker, add Weighed thioacetamide (CH 3 CSNH 2 ), stirred magnetically for 10 minutes to form solution B; introduced solution B into solution A, added sodium hydroxide (NaOH) to adjust the pH to 10; stirred magnetically for 30 minutes under the temperature condition of 80°C to fully mix, and transferred the above solution to 100mL In a stainless steel high-pressure reactor with a polytetrafluoroethylene liner, the solvothermal reaction was carried out at a temperature of 160°C, and the reaction time was 24 hours; after the product was naturally cooled to room temperature, it...
preparation example 3
[0046] According to the molar ratio of the reaction substances being basically 1:5, 1.127 g of thioacetamide powder and 0.351 g of ammonium metavanadate powder were weighed respectively. Add 30mL deionized water to a beaker, add the weighed ammonium metavanadate, stir magnetically for 30 minutes in an oil bath at 80°C, and completely dissolve to form solution A; add 30mL ethylene glycol reagent to another beaker, add Weighed thioacetamide (CH 3 CSNH 2 ), stirred magnetically for 10 minutes to form solution B; introduced solution B into solution A, added sodium hydroxide (NaOH) to adjust the pH to 12; stirred magnetically for 30 minutes under the temperature condition of 80°C to fully mix, and transferred the above solution to 100mL In a stainless steel high-pressure reactor with a polytetrafluoroethylene liner, the solvothermal reaction was carried out at a temperature of 160°C, and the reaction time was 24 hours; after the product was naturally cooled to room temperature, it...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com