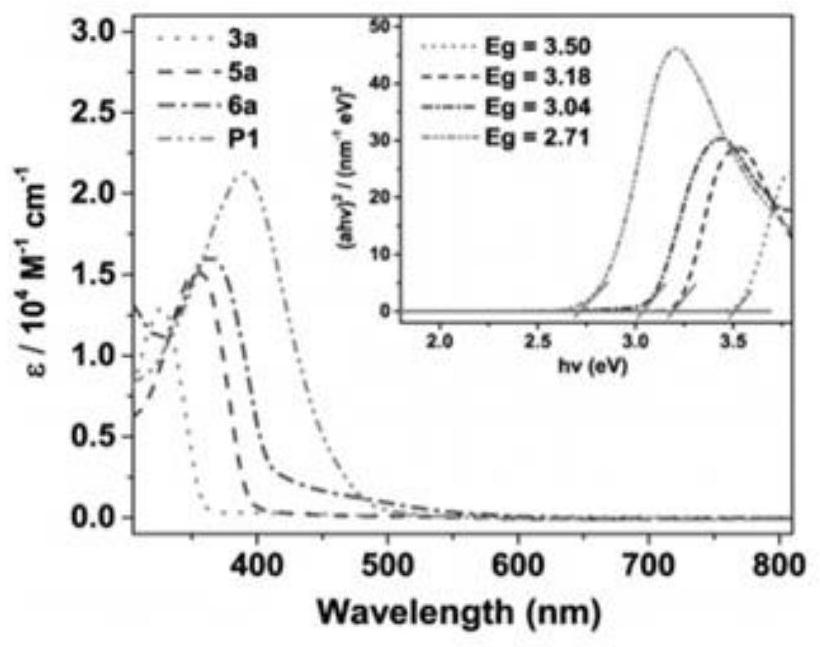
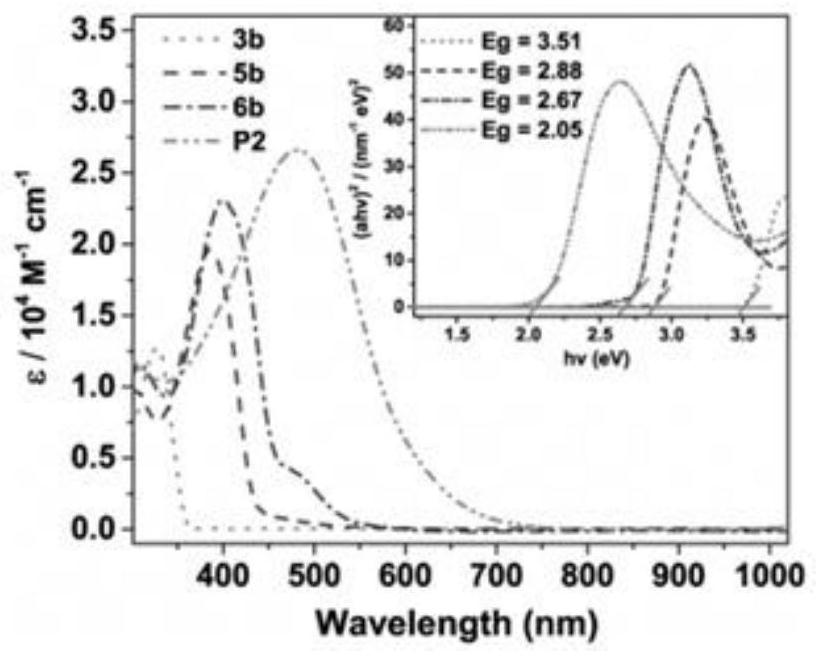
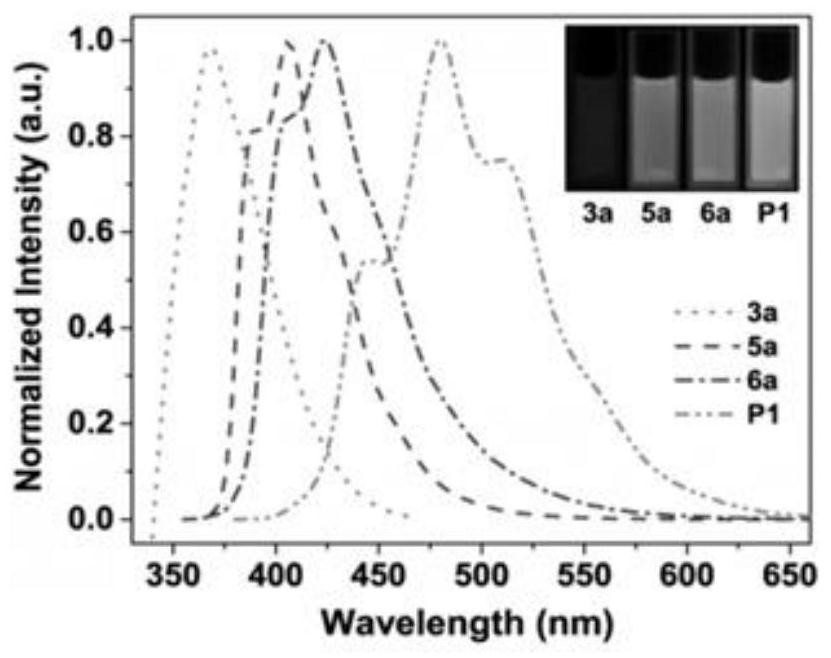
A class of borazine benzothiophene derivatives, conjugated high molecular polymers and their preparation methods and application of fluoride ion detection
A technology of thiophene derivatives and conjugated polymers, which is applied in the fields of conjugated polymers and their preparation, fluoride ion detection applications, borazine benzothiophene derivatives, and can solve problems such as hindering thiophene borazine heterocycles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0145] 1. The borazine benzothiophene small molecule and conjugated polymer described in the present invention are prepared through the following steps:
[0146] 1) Preparation of compound 2a or compound 2b
[0147] Under the protection of an inert gas, 3-bromo-2,2'-bithiophene (2.45g, 10mmol), aniline / 4-hexylaniline (11mmol), sodium tert-butoxide (1.16g, 12.10mmol), three (di Benzylideneacetone) dipalladium (293mg, 0.32mmol) and tri-tert-butylphosphine tetrafluoroborate (153.75mg, 0.53mmol) were dissolved in absolute anhydrous toluene (20mL) to obtain a reactant system; React at 100°C for 20 h, remove the solvent under reduced pressure, and separate through the column to obtain the target compound 2a or compound 2b. The reaction equation is as follows:
[0148]
[0149] 2) Preparation of compound 3a or compound 3b
[0150] Under the protection of an inert gas, the compound 2a / compound 2b (5mmol) prepared in step 1), triethylamine (1.14g, 11.25mmol), dichlorophenylborane...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com