Lidocaine cream as well as preparation method and application thereof
A lidocaine and cream technology, which is applied in the field of medicine, can solve the problems of damage to the nervous system, heart, poor skin penetration of lidocaine, increased toxicity of lidocaine, etc., and achieve the effect of avoiding side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
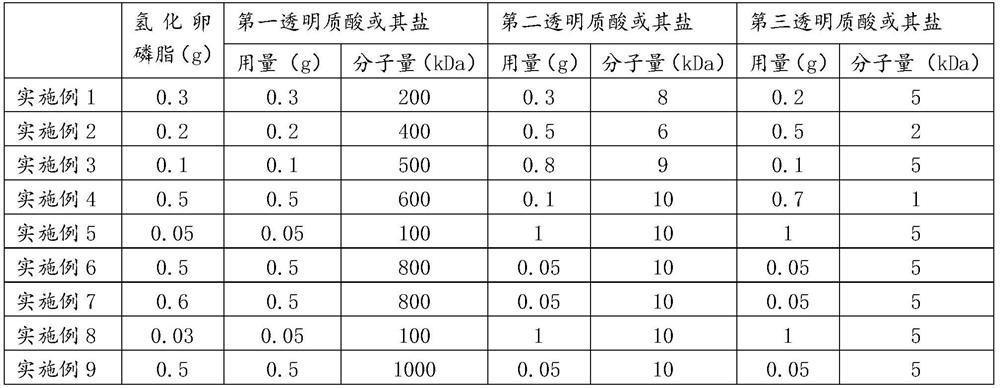
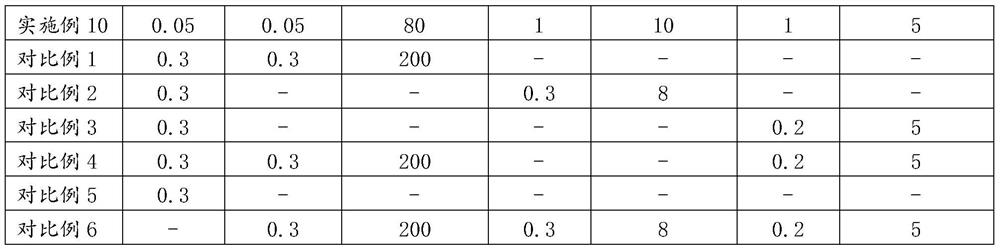
[0182] (1) 3g polyethylene glycol-6 stearate, 4g cetostearyl alcohol, 0.3g hydrogenated lecithin, 0.8g tocopheryl acetate, 9g mineral oil, 2g polydimethylsiloxane, 0.3g of methylparaben and 0.1g of ethylparaben were stirred and heated to 80°C to completely melt to obtain solution A;
[0183] (2) 4g of 1,3-propanediol, 6g of glycerin, 0.4g of xanthan gum, 0.3g of the first sodium hyaluronate with a molecular weight of 200kDa, 0.3g of the second sodium hyaluronate with a molecular weight of 8kDa, and 0.2g of a molecular weight of 5kDa third sodium hyaluronate and 0.1g allantoin were mixed and added to the remaining 85°C deionized water and stirred to dissolve to completely dissolve to obtain solution B, which was maintained at 80°C, wherein the above-mentioned The sum of the mass of components and lidocaine and benzyl alcohol is 100g;
[0184] Add solution A to solution B, keep emulsification and homogeneity at 80°C for 10 minutes, stir and cool down to 55°C, add 4g lidocaine a...
Embodiment 2
[0186] (1) Mix 6g polyethylene glycol-6 stearate, 8g cetostearyl alcohol, 0.2g hydrogenated lecithin, 1g tocopheryl acetate, 6g mineral oil, 4g polydimethylsiloxane, 0.3 g methylparaben and 0.1g ethylparaben were stirred and heated to 80°C to completely melt to obtain solution A;
[0187] (2) 5g of 1,3-propanediol, 6g of glycerin, 0.1g of xanthan gum, 0.2g of the first sodium hyaluronate with a molecular weight of 400kDa, 0.5g of the second sodium hyaluronate with a molecular weight of 6kDa, 0.5g of a molecular weight of 2kDa The third sodium hyaluronate and 0.3g allantoin were mixed and added to the remaining 80°C deionized water, stirred and heated to 80°C to completely dissolve to obtain solution B, and solution B was maintained at 75°C, wherein the above-mentioned The sum of the quality of the components and lidocaine and benzyl alcohol is 100g;
[0188] (3) Add solution A to solution B, keep emulsified and homogenized at 80°C for 10 minutes, stir and cool down to 60°C, a...
Embodiment 3
[0190] (1) 6g polyethylene glycol-6 stearate, 8g cetostearyl alcohol, 0.1g hydrogenated lecithin, 1g tocopheryl acetate, 12g mineral oil, 3g polydimethylsiloxane, 0.3 g methylparaben and 0.1g ethylparaben were stirred and heated to 80°C to completely melt to obtain solution A;
[0191] (2) 4g of 1,3-propanediol, 6g of glycerin, 0.3g of xanthan gum, 0.1g of the first sodium hyaluronate with a molecular weight of 500kDa, 0.8g of the second sodium hyaluronate with a molecular weight of 9kDa, 0.1g of a molecular weight of Mix the third sodium hyaluronate of 5kDa and 0.1g allantoin, add the remaining amount of 80°C deionized water, stir and heat to 80°C to completely dissolve to obtain solution B, and keep solution B at 85°C, wherein, the above The sum of the quality of various components and lidocaine and benzyl alcohol is 100g;
[0192] (3) Add solution A to solution B and keep it emulsified and homogeneous at 85°C for 10 minutes. After stirring and cooling down to 60°C, add 3g ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com