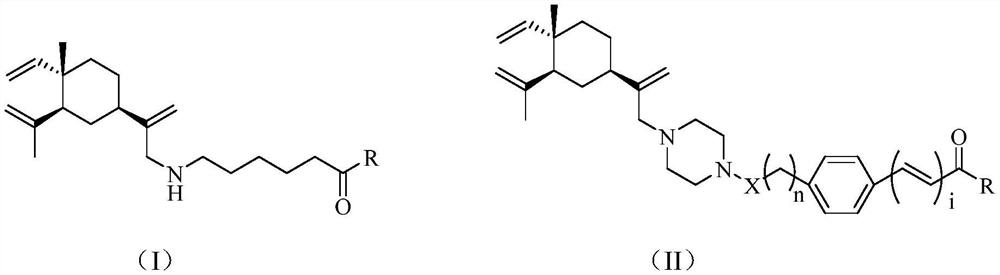
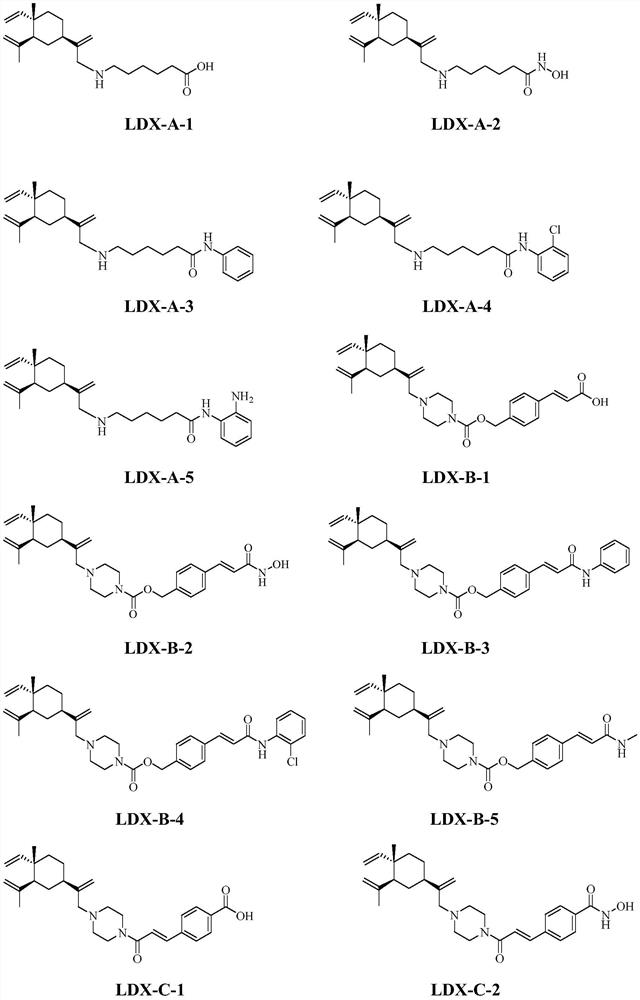
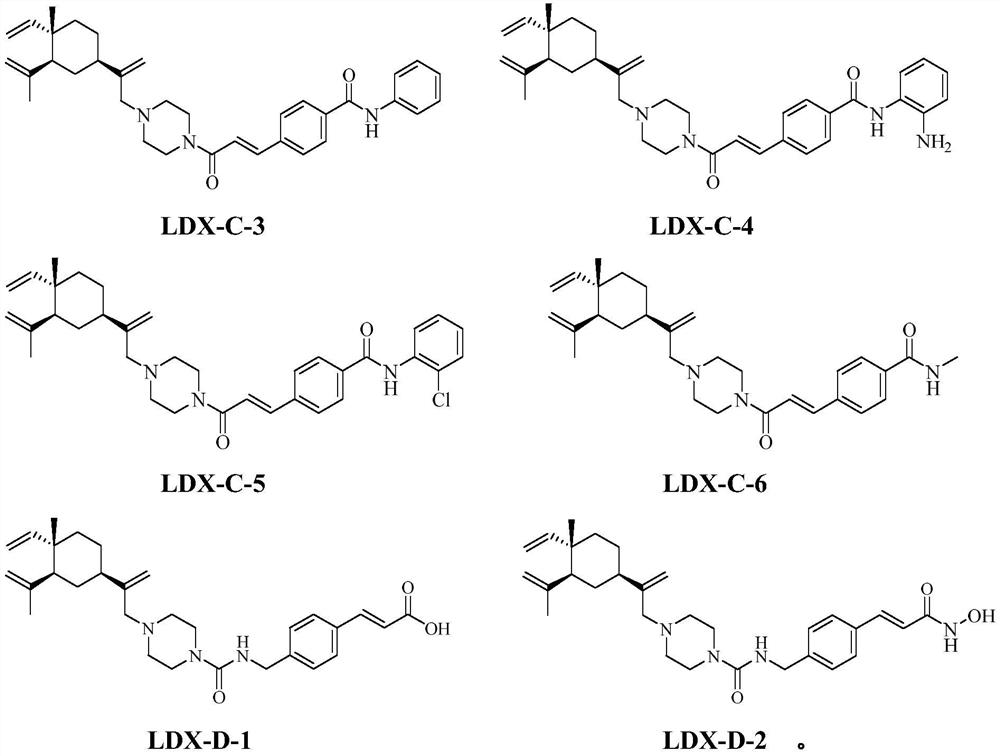
Histone deacetylase inhibitor as well as preparation and application thereof
A technology of solvate and general formula, applied in the field of histone deacetylase inhibitor and its preparation and application, can solve the problems of high toxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1 monochlorinated β-elemene intermediate preparation of
[0037] Add 5.0g of β-elemene (24.47mmol, 1eq.), 3.5mL of glacial acetic acid (61.17mmol, 2.50eq.) and 40mL of methylene chloride into a 100mL three-necked flask, cool to 5°C in an ice-water bath, and slowly Add 15mL (25.45mmol, 1.04eq.) of sodium hypochlorite solution dropwise, drop it in 15min, and continue the reaction for 4h. After the reaction, 30 mL of water was added, the aqueous layer was extracted with dichloromethane, the organic phases were combined, washed with saturated brine, and the organic layer was retained, dried over anhydrous sodium sulfate, filtered, and concentrated by distillation under reduced pressure to obtain a crude product. Column chromatography gave 2.7 g of monochlorinated β-elemene oil, with a yield of 46.2%.
Embodiment 2
[0038] Embodiment 2β-elemene piperazine preparation of
[0039] Dissolve 2.0g (8.38mmol, 1eq.) of monochlorinated β-elemene mixture and 1.8g (20.94 mmol, 2.5eq.) of anhydrous piperazine in 40mL of absolute ethanol, and react at 20-80°C for 7h. After the reaction, distill under reduced pressure, add water to the residue and extract with dichloromethane, retain the organic layer, wash the organic layer three times with saturated saline, dry over anhydrous sodium sulfate, filter, and concentrate under reduced pressure to obtain 1.2 g light yellow Oil, yield 49.7%.
Embodiment 3
[0040] Embodiment 3 intermediate 6-aminocaproic acid methyl ester hydrochloride preparation of
[0041] Add 120mL of anhydrous methanol to a 250mL three-necked flask, under nitrogen protection, lower the temperature of the reaction system to -10°C, slowly add 10.9mL of thionyl chloride (152.47mmol, 5eq.) to the reaction solution dropwise , kept at -10°C for 10 min, then added 4 g (30.49 mmol, 1 eq.) of 6-aminocaproic acid to the reaction liquid, and finally moved the reaction liquid to room temperature for 24 h. After the reaction was finished, concentrate by distillation under reduced pressure, then add 25 mL of anhydrous methanol to the residue to dissolve, add 100 mL of anhydrous ether to precipitate the product, and then filter to obtain 3.2 g of 6-aminocaproic acid methyl ester hydrochloride A1, yield 57.8% , mp: 118-121°C. ESI-MS, m / z 146.3 [M+H-Cl] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com