Alkaline anion exchange membrane for fuel cell and preparation method
An alkaline anion and fuel cell technology, applied in fuel cells, circuits, electrical components, etc., can solve problems such as poor stability of the main chain, and achieve the effects of improved conductivity, convenient operation, and high thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
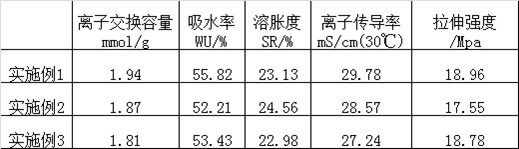
Embodiment 1
[0021] 1).Phosphazene substitution reaction: Put 12.6g of sodium metal sodium to remove surface oxide into a flask, add anhydrous tetrahydrofuran THF, pass nitrogen gas, slowly drop 54g of p-cresol anhydrous THF solution into the above flask, and reflux After reacting for 8h, sodium p-toluate nucleophile was obtained after the reaction. Put another 3.04g of sodium metal into the flask, add anhydrous tetrahydrofuran THF, blow in nitrogen gas, slowly drop 15.8g of diethylene glycol monovinyl ether anhydrous THF solution into the above flask, reflux for 8h, after the reaction Sodium diethylene glycol monovinyl ether nucleophile is obtained. Weigh 34.76g of hexachlorocyclotriphosphazene HCCP, put it into a flask and add anhydrous THF to dissolve it, slowly drop the sodium p-cresolate reagent into the flask under nitrogen, and react for 12 hours after the dropwise addition. Slowly drop the diethylene glycol monovinyl ether sodium reagent into the flask to replace the remaining P-C...
Embodiment 2
[0026] 1).Phosphazene substitution reaction: Put 12.6g of sodium metal sodium to remove surface oxide into a flask, add anhydrous tetrahydrofuran THF, pass nitrogen gas, slowly drop 54g of p-cresol anhydrous THF solution into the above flask, and reflux After reacting for 8h, sodium p-toluate nucleophile was obtained after the reaction. Put another 3.04g of sodium metal into the flask, add anhydrous tetrahydrofuran THF, blow in nitrogen gas, slowly drop 15.8g of diethylene glycol monovinyl ether anhydrous THF solution into the above flask, reflux for 8h, after the reaction Sodium diethylene glycol monovinyl ether nucleophile is obtained. Weigh 34.76g of hexachlorocyclotriphosphazene HCCP, put it into a flask and add anhydrous THF to dissolve it, slowly drop the sodium p-cresolate reagent into the flask under nitrogen, and react for 12 hours after the dropwise addition. Slowly drop the diethylene glycol monovinyl ether sodium reagent into the flask to replace the remaining P-C...
Embodiment 3
[0031] 1).Phosphazene substitution reaction: Put 12.6g of sodium metal sodium to remove surface oxide into a flask, add anhydrous tetrahydrofuran THF, pass nitrogen gas, slowly drop 54g of p-cresol anhydrous THF solution into the above flask, and reflux After reacting for 8h, sodium p-toluate nucleophile was obtained after the reaction. Put another 3.04g of sodium metal into the flask, add anhydrous tetrahydrofuran THF, blow in nitrogen gas, slowly drop 15.8g of diethylene glycol monovinyl ether anhydrous THF solution into the above flask, reflux for 8h, after the reaction Sodium diethylene glycol monovinyl ether nucleophile is obtained. Weigh 34.76g of hexachlorocyclotriphosphazene HCCP, put it into a flask and add anhydrous THF to dissolve it, slowly drop the sodium p-cresolate reagent into the flask under nitrogen, and react for 12 hours after the dropwise addition. Slowly drop the diethylene glycol monovinyl ether sodium reagent into the flask to replace the remaining P-C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com