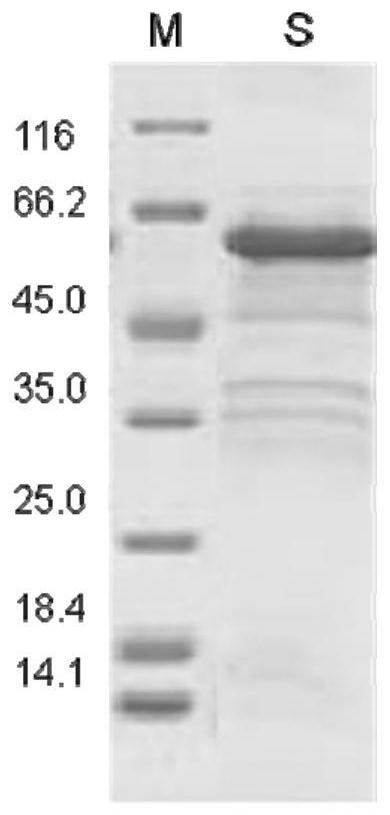
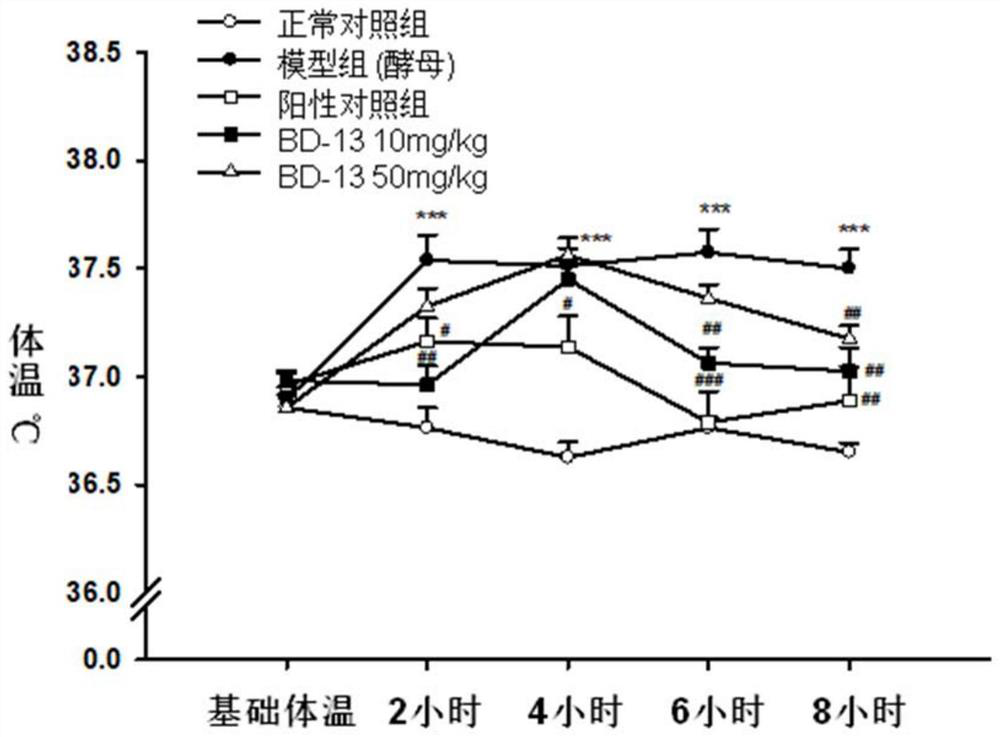
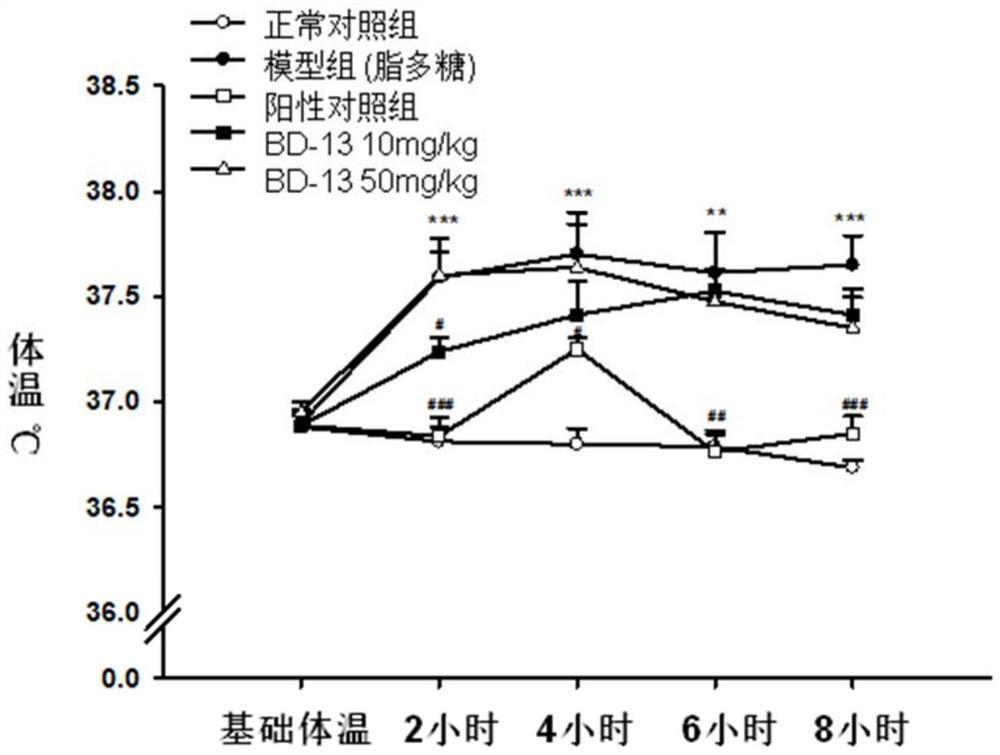
Keratin BD-13, preparation method, pharmaceutical composition and application thereof
A technology of BD-13 and keratin, applied in the direction of drug combination, keratin, cytokeratin, etc., to achieve the effect of reducing the number of writhing, high sample purity, and prolonging the onset latency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] Example 1 shake flask fermentation preparation protein BD-13 crude solution A (TB medium)
[0115] Synthesize the nucleotide sequence shown in SEQ ID No.2, and transfer it into the pET-28a (+) vector; sequencing confirms that the expression vector containing the correct sequence is obtained; the expression vector is transfected into BL21 (DE3) cells, The expression-competent host cells containing the target nucleotide sequence are obtained. Add it to LB medium, culture in a shaker at 37° C. and 220 rpm for 1 hour to obtain a recombinant strain.
[0116] The recombinant strain was dipped and streaked on the LBA plate containing Kanamycin, and the plate was placed upside down in a constant temperature incubator at 37°C for overnight cultivation for 16 hours.
[0117] Prepare 400ml of TB culture medium, divide into 2 bottles, each bottle is 200ml. Add Kanamycin (final concentration 50 μg / ml) to each bottle (200ml) of TB medium, take a single colony on the plate and add i...
Embodiment 2
[0124] Example 2 Shake flask fermentation to prepare protein BD-13 crude solution B (other medium)
[0125] Synthesized and sequenced in Example 1 to obtain an expression vector containing the sequence shown in SEQ ID No.2; the expression vector was transfected into BL21 (DE3) cells to obtain expression-competent host cells containing the target nucleotide sequence.
[0126] Prepare 20 ml of LB medium, take 800 μl, add 50 μl of host cells containing the target coding sequence, and culture in a shaker at 37° C. and 220 rpm for 1 hour.
[0127] Dip the above bacterial solution and streak it on the LBA plate containing Kanamycin, and place the plate upside down in a constant temperature incubator at 37°C for overnight cultivation for 16 hours.
[0128] Take 10 ml of LB medium, add Kanamycin (final concentration 50 μg / ml), take a single colony on the plate and add it to LB medium, in a shaker, under the conditions of 37 ° C and 220 rpm, overnight amplification culture for 15 hours...
Embodiment 3
[0134] Example 3 Fermenter preparation of protein BD-13 crude solution C
[0135] Synthesized and sequenced in Example 1 to obtain an expression vector containing the sequence shown in SEQ ID No.2; the expression vector was transfected into BL21 (DE3) cells to obtain expression-competent host cells containing the target nucleotide sequence. Add it to LB medium, culture in a shaker at 37° C. and 220 rpm for 1 hour to obtain a recombinant strain.
[0136] On the LBA plate containing Kanamycin, add 100 μl of the recombinant strain, spread it with a spreader until it dries evenly, and place the plate upside down in a constant temperature incubator at 37°C for overnight culture. Take three single colonies and streak them on a plate containing Kanamycin, culture the plate overnight, and after three batches of shake flask fermentation and expression verification confirm that it is correct, preserve the strain with 15% glycerol, and pack it into 0.8ml each to obtain Working cell bank...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com