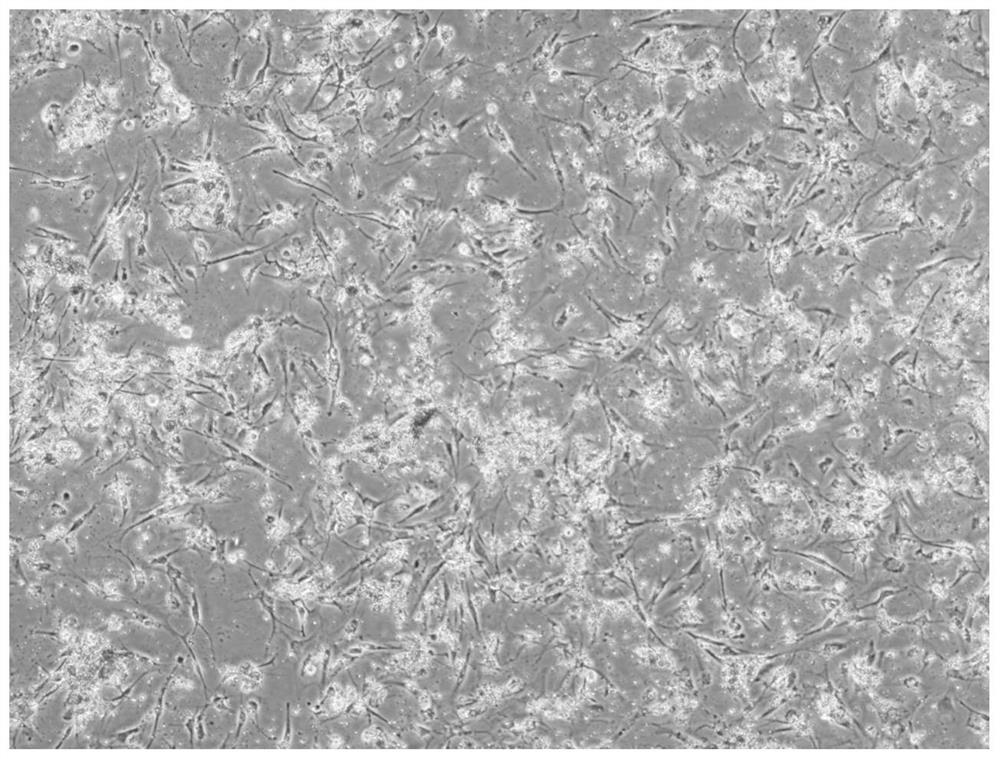
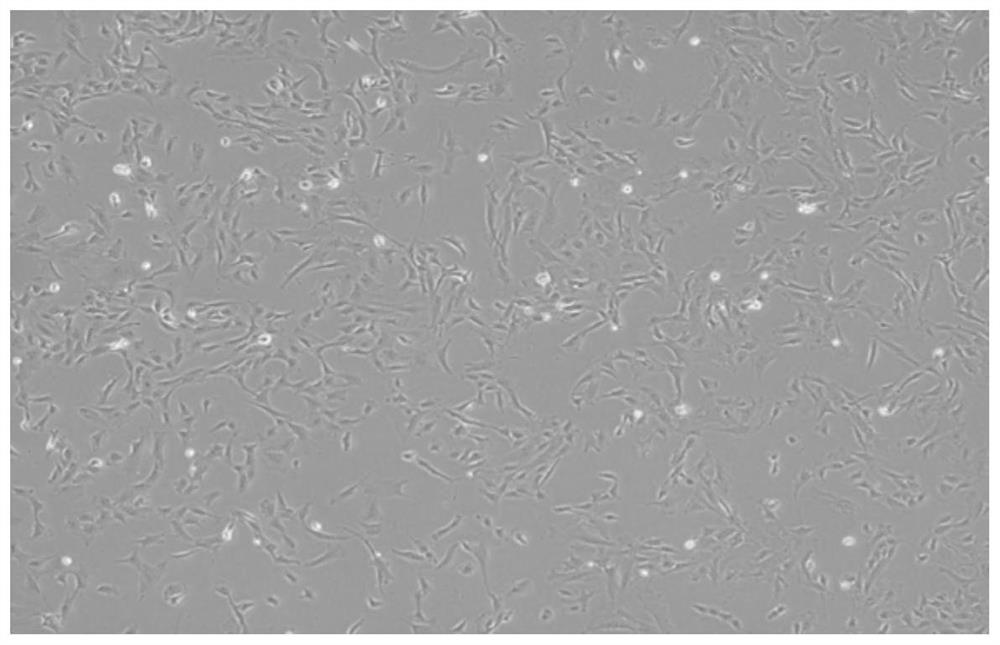
Method for establishing simulated valvular stromal cell calcification model
A technique for establishing a method and valve, applied in the field of biomedicine, which can solve the problems that the calcification pathways are not very related, and it is difficult to study the relationship in detail.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056]Example 1 and Example 2 The reagents and arrangements are as follows:
[0057]1: 50 ml of phosphate buffered saline, PBS, no calcium magnesium, Gibco, C10010500BT) + 1% penicillin-streptomycin solution (100X, Biyun Tian, C0222);
[0058]2: 15 ml of 2 mg / ml of type II collagen enzyme digestion liquid: 15 mL PBS + 30 mg Type II, Worthington, LS004176);
[0059]Reagent 3: 100 ml complete medium: 84 ml DMEM high sugar medium (Dulbecco's Modified Eaglemedium, Gibco, C11995500BT) + 15ml fetal bovine serum, FBS, Gibco, 10099141C) + 1ml 1% penicillin-streptomycin solution ;
[0060]Reagent 4: 100 ml cell digestion (GIBCO, 0.25% Trypsin-EDTA, 2186972);
[0061]Reagent 5: 100 ml of condition induced medium: 84 ml reagent 3 + 15ml fetal bovine serum + 1% penicillin-streptomycin solution;
[0062]The reagent 6: 20 ml 1.5 mg / ml of type II collagen enzyme digestion: 20 mL PBS + 30 mg II collagenase (Worthington, LS004176).
[0063]Example 1
[0064]The present invention provides a method for establishing a c...
Embodiment 2
[0093]The present invention provides a method of establishing a calcification model of a simulated valve interstitial cells for human VICS simulation calcification model, including the following steps:
[0094]S1 Prepare for the original calcified VICS
[0095]S1.1 follows my country's relevant laws and regulations to obtain a calcified valve tissue that is no longer needed by patients after CAVD patients, maintains aseptic, and placed in a pre-cooling reagent 1 to transport to the laboratory;
[0096]S1.2 rinse three times in the pre-cooled reagent 1 in S1.1 to remove blood cells, and then place each valve leaflet into a petri dish containing 3 ml reagent 2 (35 mm) In the case of 37 ° C for 30 min;
[0097]S1.3 Wipe the valve lobes in S1.2 in a sterile cotton swab (daily cotton swab high-temperature high-pressure sterilization), to ensure that each part is wiped to remove surface endocytes, will wipe The latter valve was transferred into 13 ml of reagent 2, and the sprinkled digestion overnigh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com