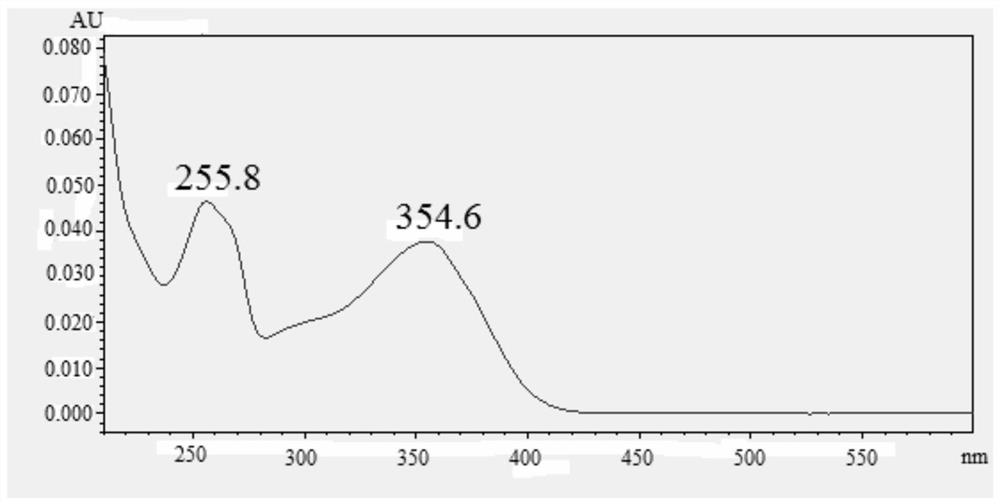
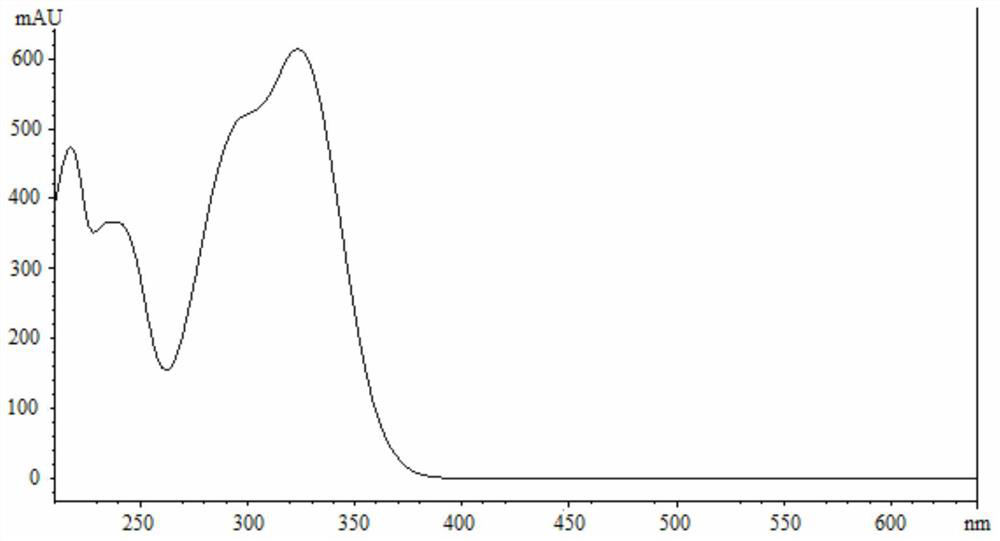
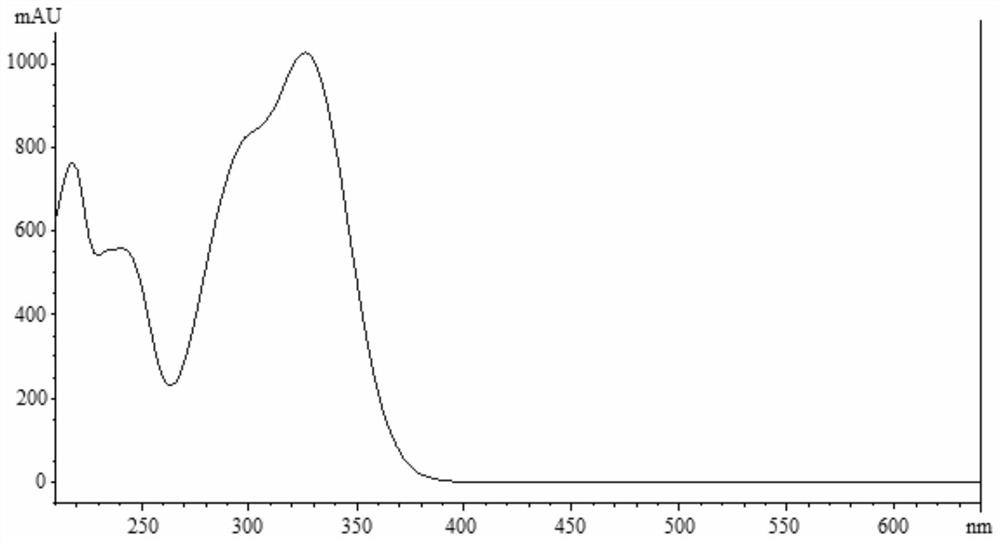
Apocynum venetum leaf formula granule characteristic spectrum identification method
A technology of characteristic maps and formula granules, which is applied in the field of identification of characteristic maps of Apocynum leaf formula granules, can solve the problems of lack, difficulty in quality monitoring of Apocynum leaf formula granules, etc., achieve high precision, simple method, and ensure the effect of drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] Preparation of reference substance solution Take hyperoside, caffeic acid, chlorogenic acid, astragalin, neochlorogenic acid, cryptochlorogenic acid, isoquercitrin reference substance amount, accurately weighed, add 70% methanol to make Each 1mL containing 30μg of the solution, that is.
[0061] Preparation of reference medicinal material solution Take 0.5 g of apocynum leaf reference medicinal material, accurately weigh, put in a stoppered Erlenmeyer flask, accurately add 50 mL of 70% methanol, seal tightly, weigh, and ultrasonically process (power 600W, frequency 40kHz) for 20 Minutes, let cool, weigh again, make up for the lost weight with 70% methanol, shake well, filter, and take the filtrate to obtain the final product.
[0062] Preparation of the test solution Take this product under the difference in loading amount, mix it evenly, take an appropriate amount, grind it finely, get 0.25g of Apocynum leaf formula granules, accurately weigh it, put it in a stoppered ...
Embodiment 1
[0148] Verification results of 3 batches of Apocynum leaf formula granules
[0149] The proposed method was used to measure the characteristic spectra of 3 batches of samples of this product, and calculate the relative retention time and relative peak area. Such as Figure 17 , Table 15 and Table 16.
[0150] Table 6 Relative retention time of 3 batches of Apocynum leaf formula granules
[0151]
[0152] Table 7 Relative peak areas of 3 batches of Apocynum leaf formula granules
[0153]
[0154] According to the principle of stable relative retention time and the detection of each batch of samples with relatively high peaks, a total of 7 peaks with good repeatability were selected as characteristic peaks. The results showed that when peak 5 was used as the S peak, the relative retention time RSDs of the characteristic peaks of the 3 batches of Apocynum leaf formula granules were between 0.01% and 0.06%, and the relative retention time RSDs of the 7 characteristic peak...
Embodiment 2
[0156] Establishment of Specified Value Limits for Relative Retention Time
[0157] See Table 17 and Table 18 for a summary of the methodological inspection items and verification results:
[0158] Table 8 Summary standard of RSD% of methodological project results—relative retention time
[0159]
[0160]
[0161] Table 18 Summary standard of RSD% of methodological project results—relative peak area
[0162]
[0163] It can be seen from the above table that the flow rate has the greatest influence on each characteristic peak, so the flow rate is set at 1mL / min, referring to the results of instrument and chromatographic column durability, in order to increase the reproducibility and applicability of the method, the relative retention time of each peak The specified value is tentatively set at 8%; and the durability and verification results have a great influence on the relative peak area, so the relative peak area is not included in the standard.
[0164] Final regul...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Column length | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com