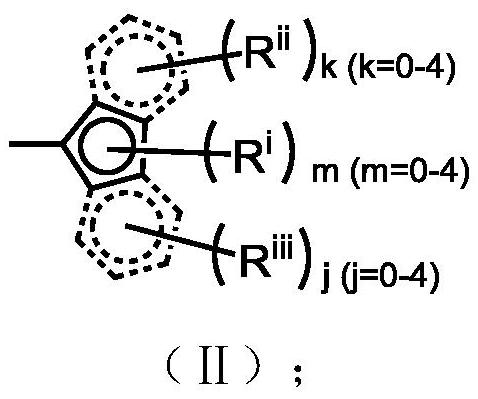
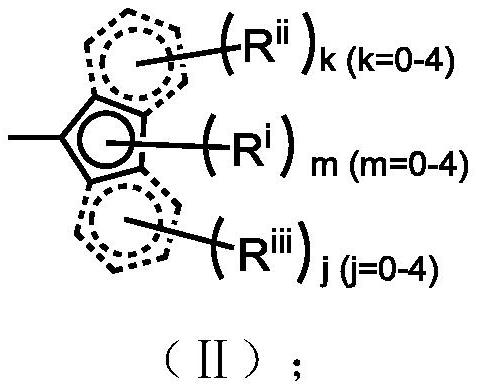
Silicon-based bridged metallocene compound as well as preparation method and application thereof
A metallocene compound and bridging technology, applied in the field of metallocene, can solve problems such as not a fixed number, and achieve good thermal stability and catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0090] The preparation of the precursor of embodiment one to eleven
[0091] Hydrogen-based silicon-bridged bis-indenyl zirconocene compound MeHSi (2-Me-7-p-tBuC 6 h 4 C 9 h 4 ) 2 ZrCl 2 Preparation of (MS-1).
[0092] Weigh 2-methyl-7-p-tert-butylphenylindene (5.24g, 20mmol), dissolve in Tol (80ml) solvent, slowly add n-butyl lithium (2.4M, 8.5mL, 20mmol), gradually return to room temperature and react overnight to obtain a wine red solution. Methyldichlorosilane (1.04 mL, 10 mmol) was slowly added dropwise at -78°C, and gradually returned to room temperature and stirred for more than 8 hours to obtain a yellow suspension. The yellow suspension was placed at -78°C, and n-butyllithium (2.4M, 8.5mL, 20mmol) was slowly added dropwise. After returning to room temperature, stirring was continued for 2h to obtain an orange-yellow turbid solution. Take zirconium tetrachloride (2.33g, 10mmol) in a small bottle in the glove box, add 40mL of toluene, take out the nitrogen prote...
Embodiment 1
[0095] Amino-alkyl-silicon-bridged bis-indenyl zirconium compounds Me(Me 2 NCH 2 CH 2 )Si(2-Me-7-p-tBuC 6 h 4 C 9 h 4 ) 2 -ZrCl 2 Preparation of (rac-MS-1a)
[0096] Weigh rac-MS-1 (1.45g, 2mmol), dissolve in Tol (100ml) solvent, add Me 2 NCH=CH 2 (0.156g, 2.2mmol) and B(C 6 f 5 ) 3 (0.051g, 0.1mmol, 5% usage), heated to 50°C for 24h. All volatile components were removed by vacuuming at room temperature, and the remaining solid was washed 2 to 4 times with a small amount (about 1.5 mL each time) of n-hexane. After vacuum drying for 6 h, 1.36 g (85.2%) of rac-MS-1a was obtained as a yellow solid.
[0097] composed of C 45 h 57 Cl 2 NSiZr (Mr=802.16): Theoretical: C, 67.38; H, 7.16; N, 1.75; Found: C, 67.42; H, 7.19; N, 1.78.
Embodiment 2
[0099] Amino-alkyl-silicon-bridged bis-indenyl zirconium compounds Me(Me 2 NCH 2 CH 2 )Si(2-Me-7-p-tBuC 6 h 4 C 9 h 4 ) 2 -ZrCl 2 Preparation of (meso-MS-1a)
[0100] The implementation steps were the same as in Example 1, wherein meso-MS-1 (1.45 g, 2 mmol) was used instead of rac-MS-1, and finally 1.4 g (87.7%) of yellow solid meso-MS-1a was obtained.
[0101] The compound meso-MS-1 and the above rac-MS-1 are isomers, and the composition is also C 45 h 57 Cl 2 NSiZr (Mr=802.16): Theoretical: C, 67.38; H, 7.16; N, 1.75; Found: C, 67.44; H, 7.18; N, 1.77.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Polymerization activity | aaaaa | aaaaa |
| Polymerization activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com