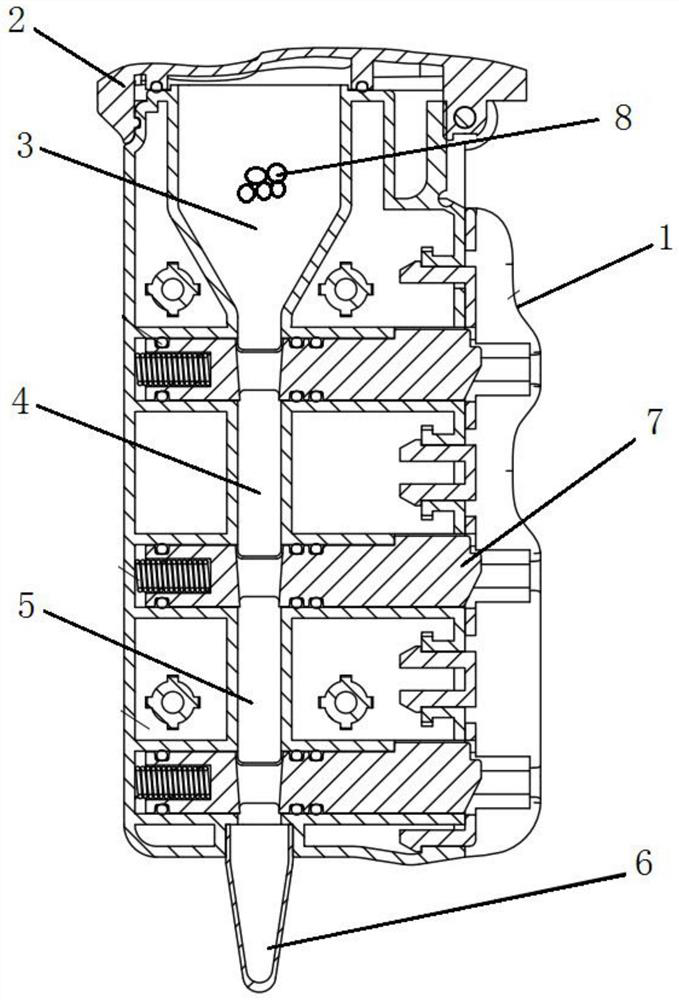
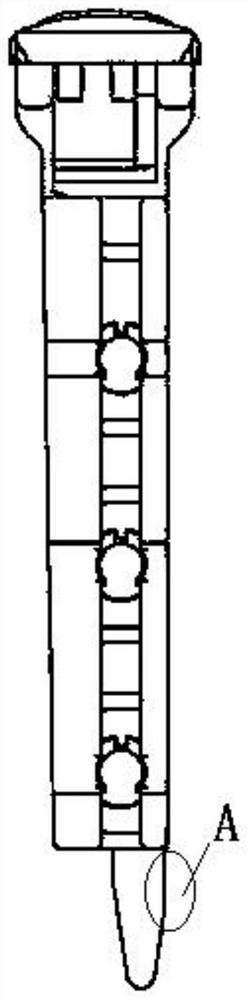
Novel coronavirus integrated nucleic acid rapid detection card box
Technology of a coronavirus, test card, used in the field of medical testing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 Primer probe design and screening.
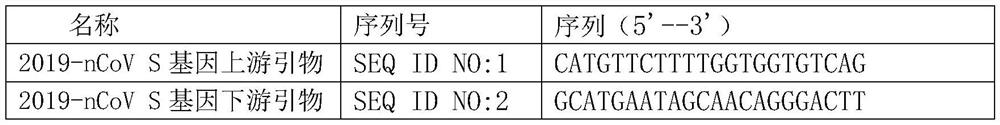
[0040] 1. The present invention analyzes and studies the sequence of the novel coronavirus 2019-nCoV, and designs the primer probe sets shown in Table 1-Table 4.
[0041] Table 1 Primer probe of the present invention
[0042]
[0043]
[0044] Table 2 Control group 1 Primer probe
[0045]
[0046] Table 3 control group 2 primer probe
[0047]
[0048]
[0049] Table 4 Control group 3 primer probes
[0050]
[0051] 2. At the same time, through the optimization of the detection system, the following detection system was established: add 30ul RT-PCR amplification solution and 5ul manganese ion solution to each PCR tube. And add 5ul template RNA.
[0052] 30ul RT-PCR amplification solution: including primers, probes and PCR buffer;
[0053] The concentration of the probe used to detect the S target gene is 200nM, the concentration of the probe used to detect the N target gene is 200nM, the concentration ...
Embodiment 2
[0064] Example 2. Creation of reagent cartridges.
[0065] 1. The reagent cartridge includes the following components:
[0066] ①Primer probe combination
[0067]
[0068] ②Detection solution components:
[0069] The composition of PCR buffer is: 50mmol / L Tris-HCl (pH8.0), 20mmol / L (NH 4 ) SO 4 , 30mmol / LKCl, 4.0mmol / L MgCl 2 , 0.2mmol / L dNTPs, 5U rTth DNA polymerase, the concentration of the probe used to detect the S target gene is 200nM, the concentration of the probe used to detect the N target gene is 200nM, the concentration of the probe used to detect the internal standard is 100nM, used for The concentration of upstream and downstream primers used to detect the S target gene is 400nM, the concentration of upstream and downstream primers used to detect the N target gene is 400nM, and the concentration of upstream and downstream primers used to detect the internal standard is 200nM;
[0070] 5ul manganese ion solution (25mM manganese acetate);
[0071] ③ Purifi...
Embodiment 3
[0099] Example 3. Test Example 1.
[0100] 1. In order to test the accuracy and effectiveness of the cartridge of the present invention for the detection of 2019-nCoV, we tested the sensitivity and specificity of the cartridge.
[0101] 1.1 Test the 2019-nCoV quality control products at different concentrations to confirm the detection limit;
[0102] 1.2 For endemic human coronaviruses (HKU1, OC43, NL63 and 229E), SARS coronavirus, MERS coronavirus, H1N1, H3N2, H7N9, influenza B, respiratory syncytial virus, parainfluenza virus types 1, 2, and 3, Adenovirus, rubella virus, vesicular stomatitis virus, Haemophilus influenzae, Staphylococcus aureus, Streptococcus pneumoniae, Mycobacterium tuberculosis, Candida albicans, Candida glabrata and human genome were tested to verify specificity
[0103] 2. Results:
[0104] 2.1 We tested different concentrations of 2019-nCoV quality control products, and the following 2 tables are the results of S gene and N gene respectively.
[010...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com