Bismuth ferrite/sepiolite composite visible-light-driven photocatalyst and preparation method thereof
A technology of sepiolite and bismuth ferrite, applied in the field of photocatalytic degradation of organic pollutants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
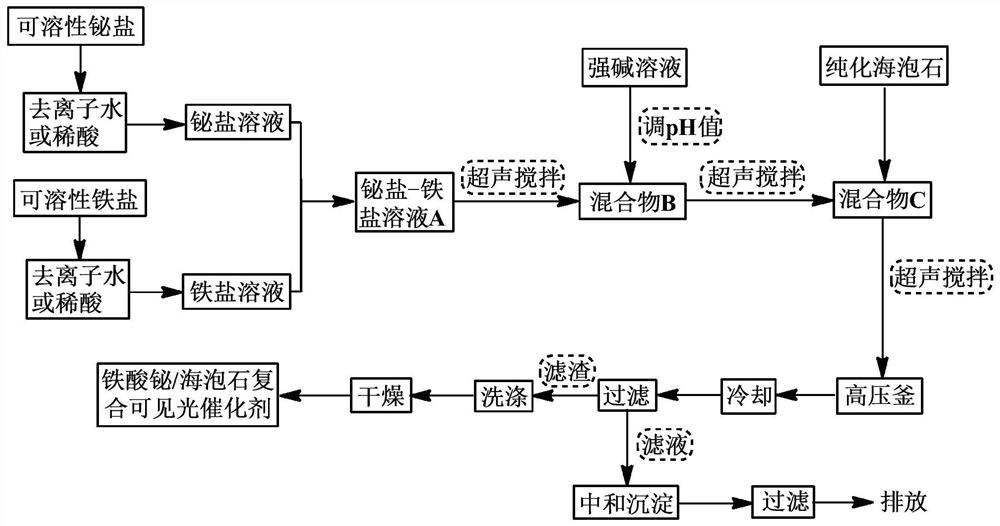
[0033] (1) get 2.70g content and be that 99.0% bismuth nitrate pentahydrate is dissolved in 110mL deionized water to prepare Fe 3+ Concentration is 0.050mol / L solution, then take 2.26g ferric nitrate content is 98.5% and dissolve in 122mL deionized water to prepare Bi 3+ The concentration is 0.045mol / L solution; the two solutions are mixed, ultrasonically stirred for 2 hours, and bismuth nitrate-ferric nitrate solution A is obtained.
[0034] (2) Adjust the pH to 13.5 with 8 mol / L KOH solution, and continue ultrasonic stirring at room temperature for 2 hours to obtain mixture B;
[0035] (3) Add 0.27g of purified sepiolite to mixture B, and ultrasonically stir for 1.5h to obtain mixture C;
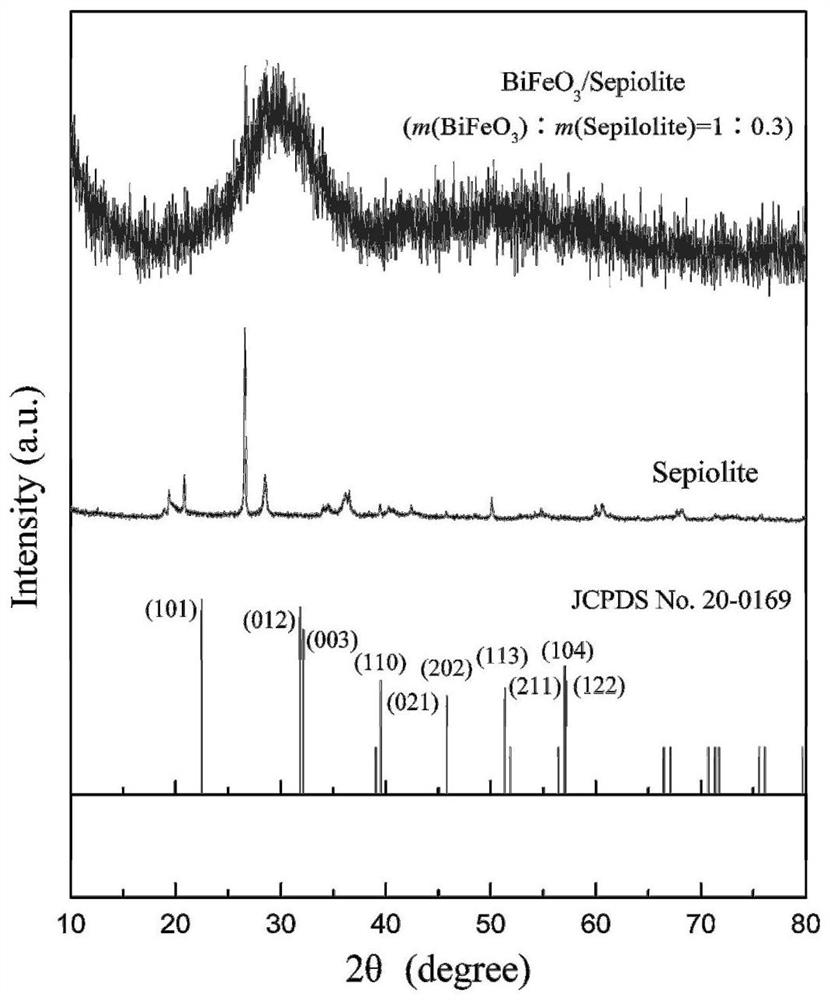
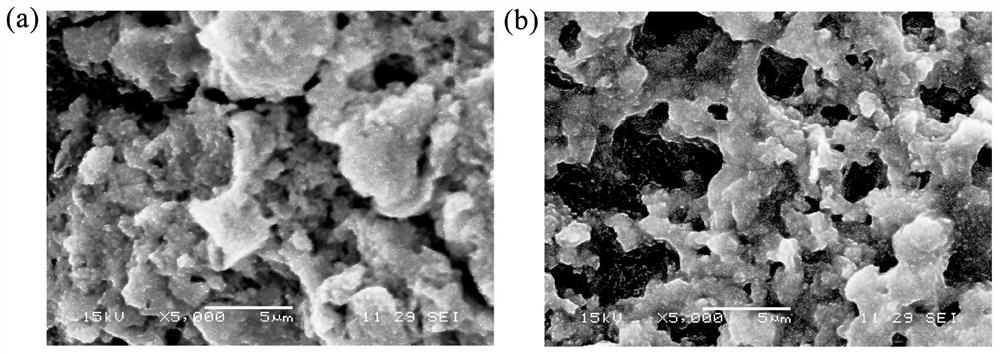
[0036] (4) Transfer the mixture C prepared in step (3) into a high-pressure reactor, and the reaction time at 200°C is 6h; cool to room temperature, filter, and wash the filter residue 3 times with deionized water and ethanol respectively, and heat it at 80°C After drying to constant wei...
Embodiment 2
[0039] (1) Get 2.70g of bismuth nitrate pentahydrate with a content of 99.0% and dissolve it in 100mL0.1mol / L dilute nitric acid to prepare Bi 3+ The concentration is 0.055mol / L solution, then take 2.21g ferric nitrate with a content of 98.5% and dissolve it in 108mL0.1mol / L dilute nitric acid to prepare Fe 3+ The concentration is 0.050 mol / L solution; the two solutions are mixed and ultrasonically stirred for 1.5 hours to obtain bismuth nitrate-ferric nitrate solution A.
[0040] (2) Use 8mol / L KOH solution to adjust the pH to 13.0, and continue ultrasonic stirring at room temperature for 1.5h to obtain mixture B;
[0041] (3) Add 0.22 g of purified sepiolite to mixture B, and ultrasonically stir for 1.5 h to obtain mixture C;
[0042] (4) Transfer the mixture C prepared in step (3) into a high-pressure reactor, and the reaction time is 7h at 190°C; cool to room temperature, filter, and wash the filter residue 4 times with deionized water and ethanol, and then wash it at 75°...
Embodiment 3
[0045] (1) 2.70g content of 99.0% bismuth nitrate pentahydrate was dissolved in 92mL deionized water to prepare Bi 3+ Concentration is 0.060mol / L solution, then take 2.21g ferric nitrate with content of 98.5% and dissolve it in 95mL deionized water to prepare Fe 3+ The concentration is 0.055mol / L solution; the two solutions are mixed and stirred ultrasonically for 1 hour to obtain bismuth nitrate-ferric nitrate solution A.
[0046] (2) Adjust the pH to 12.6 with 6mol / L KOH solution, and continue ultrasonic stirring at room temperature for 1 hour to obtain mixture B;
[0047] (3) Add 0.54 g of purified sepiolite to mixture B, and ultrasonically stir for 1 hour to obtain mixture C;
[0048] (4) Transfer the mixture C prepared in step (3) to a high-pressure reactor, and the reaction time is 8h at 180°C; cool to room temperature, filter, and wash the filter residue 4 times with deionized water and ethanol, Dry to constant weight to obtain 2.20 g of bismuth ferrite / sepiolite comp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com