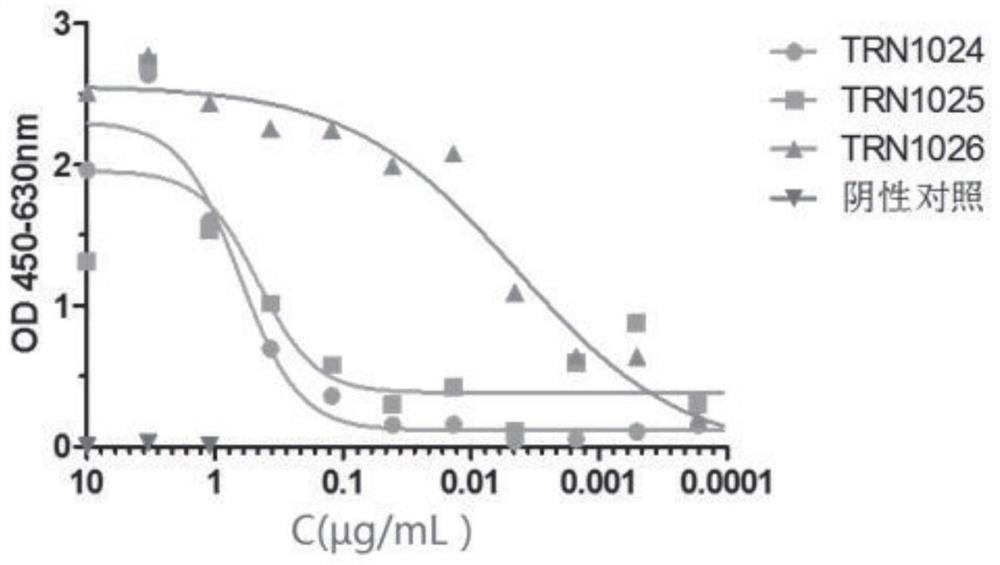
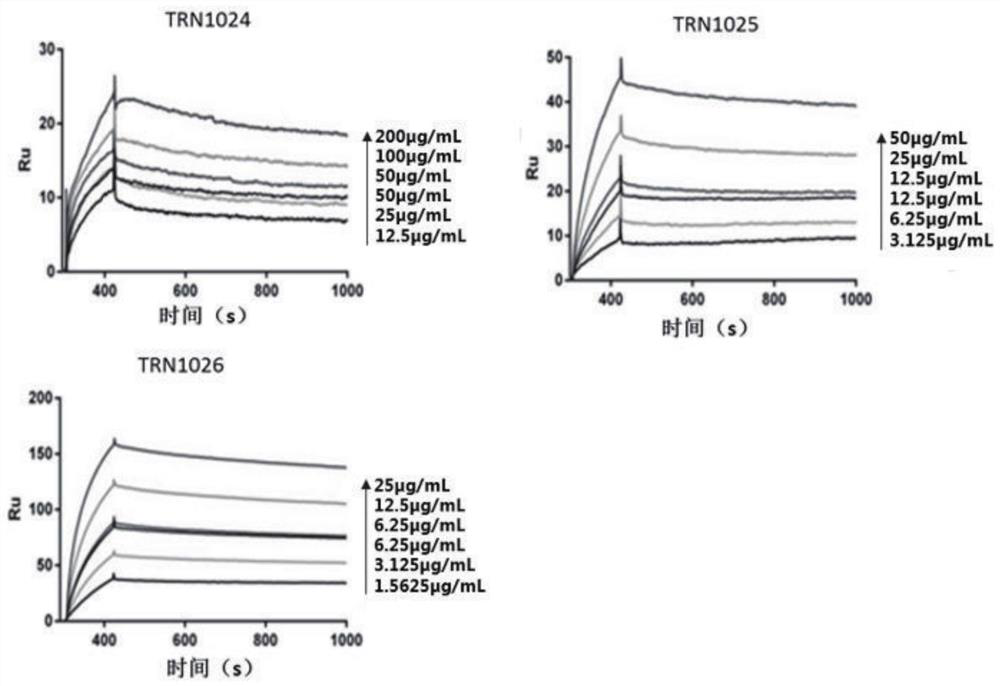
Antibody against varicella-zoster virus
A technology of herpes zoster virus and chickenpox, applied in the direction of antiviral agent, antiviral immunoglobulin, antibody, etc., can solve the problem of inability to eliminate the complications of VZV infection, achieve no xenogeneic serum reaction, strong specificity and good affinity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] Example 1 Plasma Cell Sorting
[0103] Volunteers were inoculated with varicella vaccine (Merck, Zostavax) according to the manufacturer's method, and blood samples were collected on the 7th day after inoculation, and plasma and PBMC cells were separated by density centrifugation. For specific methods, please refer to the invention patent with the authorized announcement number CN107760690B . The VZV gH / gL protein complex (CAMBRIDGEBIO, Cat No: 01-11-0045) was selected as the antigen for serum antibody titer ELISA detection, and the sample with the highest fold change in antibody titer was screened out (dilution 50 times, OD value >2.0) for flow sorting. By flow cytometry, single plasma cells were sorted by CD3 / CD14 / CD16 / cd235a-CD19+CD20+ / -CD38hi CD27hi gating, and the specific plasma cell population was isolated, and the fully human monoclonal anti-VZV was isolated from them For the gene sequence of the antibody, please refer to the invention patent with the authoriz...
Embodiment 2
[0104] Example 2 Isolation of the variable region gene of the antibody of interest
[0105] The first strand of cDNA was synthesized by reverse transcription from the plasma cells obtained in Example 1 using constant region primers (see primer information disclosed in CN107760690B) and Superscript III reverse transcriptase (Invitrogen, Carlsbad, CA). The antibody gene was then isolated by the following PCR procedure: the first round of PCR: 50ul system contained 5ul of reverse transcription reaction product, 5 units of Taq enzyme, 0.2mM dNTPs, and 0.5uM of heavy chain and light chain of each subtype Antibody constant region primer (sequence), reaction conditions: pre-denaturation at 95°C for 5min, then 35 PCR cycles, each cycle: 95°C×30s, 55°C×60s, 72°C×90s, and finally extended at 72°C for 7min . The second round of PCR: 50ul system contains 2.5ul of the first round of PCR reaction product, 5 units of Taq Plus enzyme, 0.2mM dNTPs, and 0.5uM of heavy chain and light chain ant...
Embodiment 3
[0107] Embodiment 3 constructs the expression of recombinant antibody
[0108] The obtained PCR product of antibody variable region gene was connected to the pcDNA3.3 vector containing human IgG1 constant region by TA cloning method to construct the expression vector of fully human neutralizing antibody against varicella-zoster virus, and then The expression vector was transformed into DH5α competent bacteria for vector amplification and recombinant plasmid was extracted. Co-transfect HEK293 cells with the obtained recombinant plasmid and the transfection reagent PolyFect, 37°C, 8% CO 2 Cultured in an incubator, the paired heavy chain and light chain gene expression vectors were expressed in the cells, the supernatant was collected after 96 hours of culture, the cell debris was discarded by centrifugation, and the supernatant was purified by Protein A affinity chromatography. The purified antibody was tested by SDS-PAGE, and the results were as follows: figure 1 It shows tha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com