Method for preparing isononanal through diisobutylene hydroformylation
A technology of diisobutene hydroformyl and diisobutene, which is applied in the directions of carbon monoxide reaction preparation, carbon-based compound preparation, chemical instruments and methods, etc., can solve the problems of low conversion rate of olefins, low yield of isononanal, etc., and achieve the synthesis cost. Low, rich source of raw materials, good effect of catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
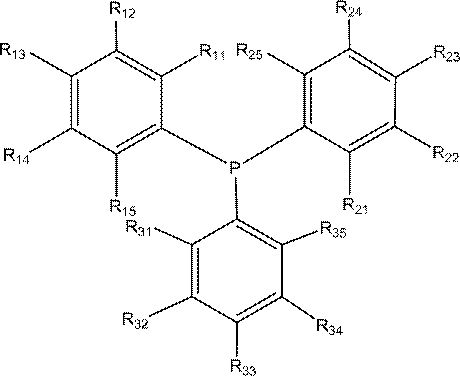
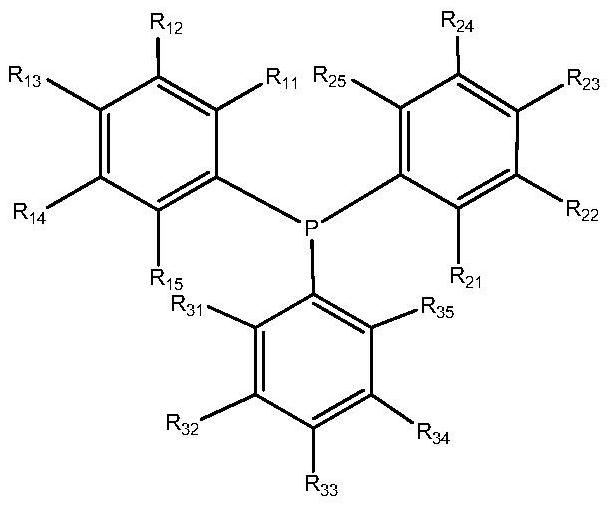
[0035] Weigh a certain amount of Rh(acac)(CO) 2 and ligand L A Tris(3,5-dimethoxyphenyl)phosphine and Ligand L B Phosphite tris (2-tert-butyl-4-methoxyphenyl) ester, then add the 100ml self-control high-pressure reactor that 30ml solvent toluene is housed, start stirring and generate catalyst solution, make the rhodium concentration in the solution be 250mg / Kg, Phosphine / rhodium molar ratio 20:1, organophosphine ligand L A and L B The molar ratio is 4:1. Connect the gas line, introduce nitrogen to replace the gas in the kettle three times, and then introduce 0.35g / (ml solvent) diisobutene (containing 75% of the mass fraction of 4,4,2-trimethyl-1-pentene and 25 % of 4,4,2-trimethyl-2-pentene), the reaction kettle is heated up to the set temperature (100°C), the stirring speed is set at 500 rpm, and after the temperature of the reaction system is stable, hydrogen gas is introduced Mixed gas with carbon monoxide (1:1) to a total pressure of 1.2MPa, after 4 hours of constant ...
Embodiment 2
[0037] Carry out diisobutylene hydroformylation reaction by the method for embodiment 1, and its difference is that organophosphine ligand L A and L B The molar ratio was changed to 0.1:1, and the reaction results are listed in Table 1.
Embodiment 3
[0039] Carry out diisobutylene hydroformylation reaction by the method for embodiment 1, and its difference is that organophosphine ligand L A and L B The molar ratio of the compound was changed to 10:1, and the reaction results are listed in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com