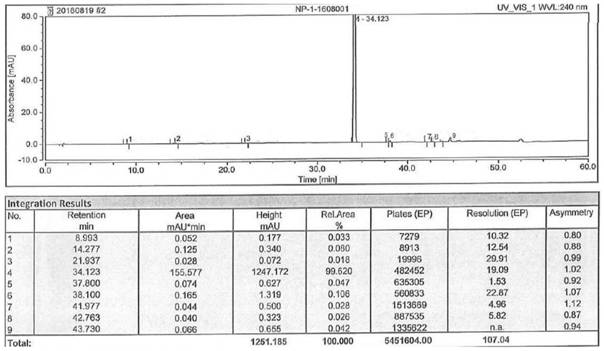
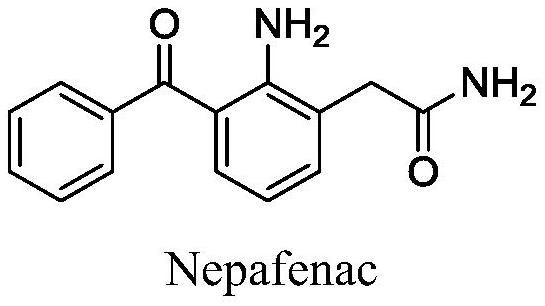
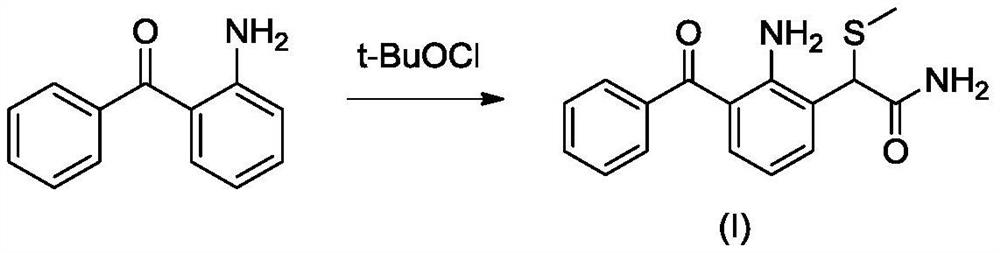
Preparation method of high-purity nepafenac intermediate
An intermediate, acetamide technology, applied in the field of pharmaceutical synthesis, can solve the problems of defective refining methods, harsh reaction conditions, low reaction temperature, etc., and achieve the effects of easy control, rapid reaction and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050]S1: 2-amino-3-benzoyl-α- (methylthio) phenylacetamide synthesis
[0051]67.70 g of N-chlorobutylimide was dissolved in 1000 ml of dichloromethane, and then 100 g 2-aminobenzophenone was dissolved in 1000 ml of dichloromethane. The dichloromethane solution of 2-aminobenzodone was slowly added dropwise to the dichloromethane solution of 2-aminobenzodone at -35 ° C ~ -25 ° C. After the dropwise addition was completed, the reaction was maintained at -35 ° C ~ -25 ° C for 0.5 h. After the time, 53.32 g 2- (methylthio) acetamide was slowly added to the reaction system, raised the temperature, and the control temperature was reacted at 0 ° C to 10 ° C for 2 h. TLC determines the end point of the reaction, the expander is VPetroleum ether : VEthyl acetate = 2: 1. After the reaction was completed, 56.43 g of triethylamine was slowly added dropwise at temperatures at 0 ° C ~ 10 ° C. After the dropwise addition, the entire system became a white turbidity into orange red clarified and transp...
Embodiment 2
[0056]S1: 2-amino-3-benzoyl-α- (methylthio) phenylacetamide synthesis
[0057]67.70 g of N-chloroimide was dissolved in 1000 ml of tetrahydrofuran in 1000 ml of tetrahydrofuran, and then dissolved in 1000 ml of tetrahydrofuran. At -35 ° C ~ -25 ° C, 2-aminobenzophenone tetrahydrofuran solution was slowly added to the tetrahydrofuran of N-chloroimide. After the dropwise addition was completed, the reaction was maintained at -35 ° C ~ -25 ° C for 0.5 h. After the time, 53.32 g 2- (methylthio) acetamide was slowly added to the reaction system, raised the temperature, and the control temperature was reacted at 0 ° C to 10 ° C for 2 h. TLC determines the end point of the reaction, the expander is VPetroleum ether : VEthyl acetate = 2: 1. After the reaction was completed, 56.43 g of triethylamine was slowly added dropwise at temperatures at 0 ° C ~ 10 ° C. After the dropwise addition, the entire system became a white turbidity into orange red clarified and transparent solution. Add pure wate...
Embodiment 3
[0061]S1: 2-amino-3-benzoyl-α- (methylthio) phenylacetamide synthesis
[0062]67.70 g of N-chlorobutyimide was dissolved in 1000 ml of ethyl acetate, and 100 g 2-aminobenzophenone was taken from 1000 ml of ethyl acetate. At -35 ° C ~ -25 ° C, 2-amino diphenyl acetate solution slowly droplets in ethyl acetate solution of N-chloroimide. After the dropwise addition was completed, the reaction was maintained at -35 ° C ~ -25 ° C for 0.5 h. After the time, 53.32 g 2- (methylthio) acetamide was slowly added to the reaction system, raised the temperature, and the control temperature was reacted at 0 ° C to 10 ° C for 2 h. TLC determines the end point of the reaction, the expander is VoilEther: VEthyl acetate = 2: 1. After the reaction was completed, 56.43 g of triethylamine was slowly added dropwise at temperatures at 0 ° C ~ 10 ° C. After the dropwise addition, the entire system became a white turbidity into orange red clarified and transparent solution. Add pure water 500 ml to the reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com