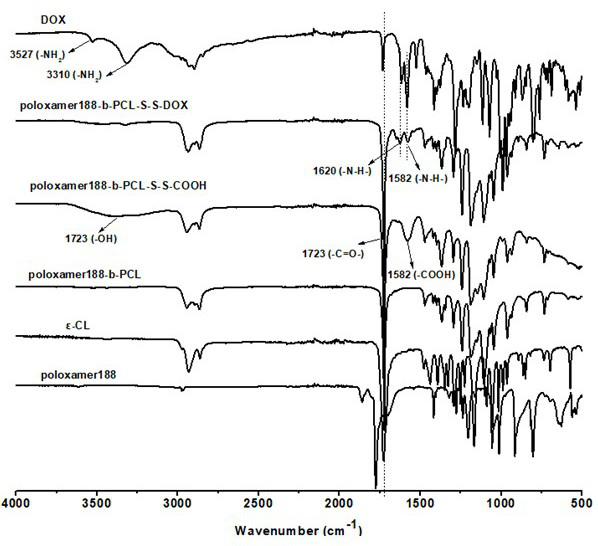
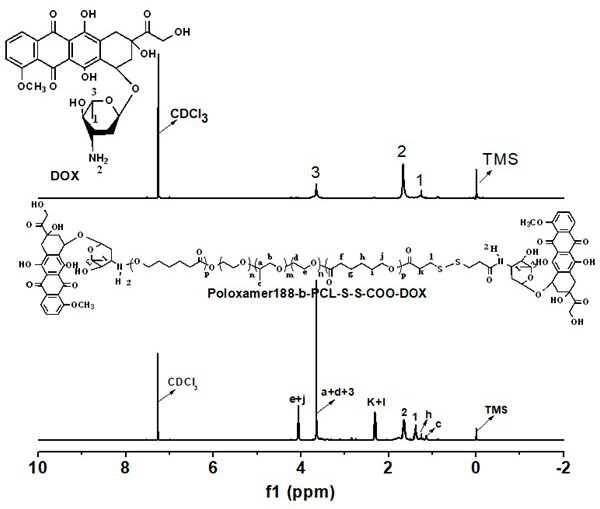
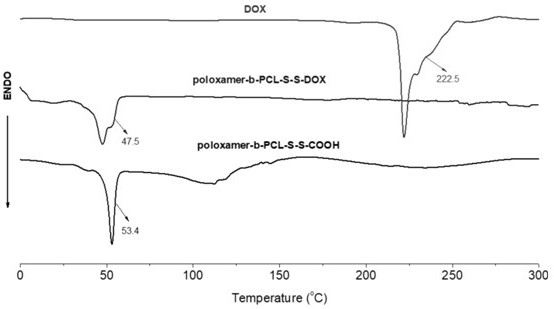
Disulfide bond-containing redox sensitive drug delivery system as well as preparation method and application thereof
A delivery system and sensitive technology, applied in the field of redox-sensitive drug delivery system and its preparation, can solve problems such as fracture
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-6
[0051] Polymer Poloxamer-b-PCL was prepared by ring-opening polymerization. With Poloxamer188 as the initiator, stannous octoate (Sn(Oct) 2 ) or tin triflate (Sn(oTf) 2 ) is a catalyst, which is prepared by reacting under anhydrous and oxygen-free conditions, and specific examples are as follows:
[0052]
Embodiment 1
[0054] Add 1.6g (0.2mmol) dry poloxamer188 (number average molecular weight 8000), 1.6g (14.0mmol) ε-caprolactone and 200μL stannous octoate into the dry reaction tube, vacuumize, fill with nitrogen, and repeat the operation three times. It was then placed in an oil bath at 140° C. for 10 h and then terminated. Finally, the product was dissolved in 6 mL of dichloromethane, then precipitated in glacial ether, and the product was obtained by suction filtration, and vacuum-dried at room temperature for 24 hours to obtain the polymer Poloxamer188-b-PCL.
Embodiment 2
[0056] Add 1.6g (0.2mmol) dry poloxamer188 (number average molecular weight 8000), 3.2g (28.0mmol) ε-caprolactone and 200μL stannous octoate into the dry reaction tube, vacuumize, fill with nitrogen, and repeat the operation three times. It was then placed in an oil bath at 140° C. for 10 h and then terminated. Finally, the product was dissolved in 6 mL of dichloromethane, then precipitated in glacial ether, and the product was obtained by suction filtration, and vacuum-dried at room temperature for 24 hours to obtain the polymer Poloxamer188-b-PCL.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com