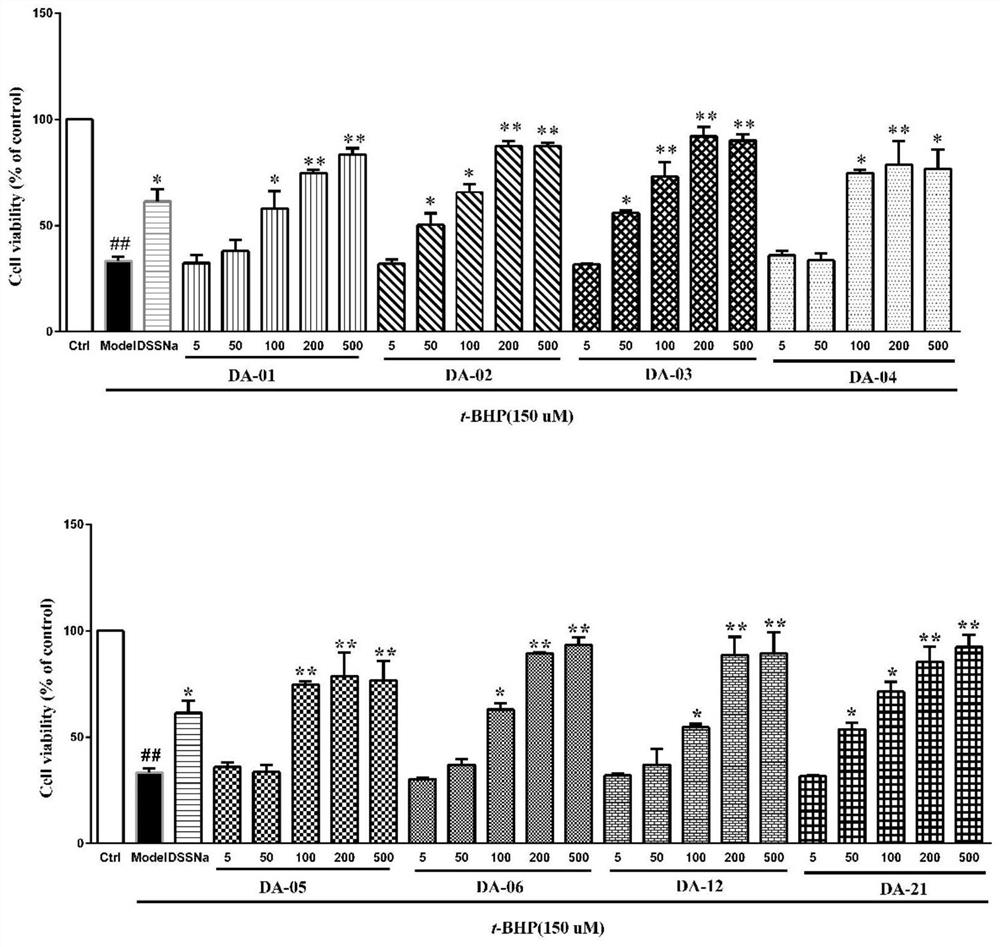
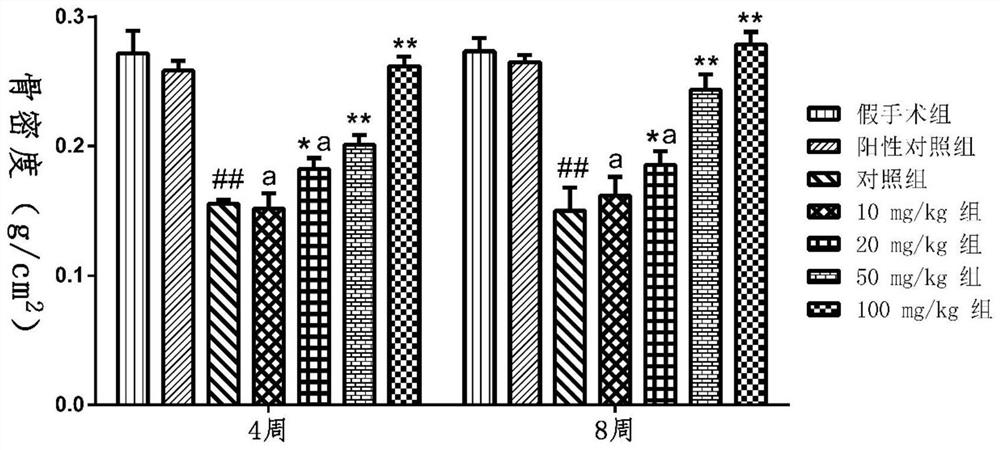
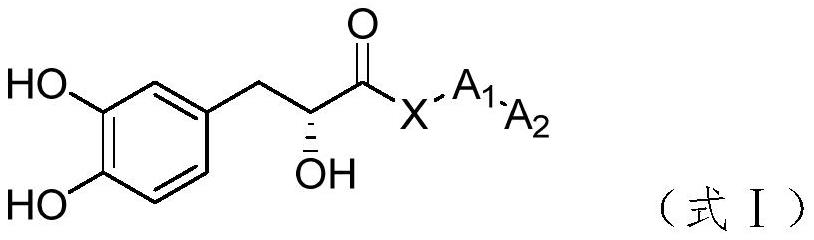
Tanshinol derivative and preparation method and medical application thereof
A Danshensu derivative and pharmaceutical technology, applied in the field of Danshensu derivatives and its preparation, can solve the problems of irritation, intestinal mucosal damage, and DSS cycle time not being improved, and achieve easy synthesis, reasonable design, and significant protective effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] The synthesis of embodiment 1 compound DA-01
[0073]
[0074] The specific synthesis is as follows:
[0075]Danshensu sodium (200mg, 0.909mmol) and 8ml of anhydrous N,N-dimethylformamide (DMF) were added into a 25ml single-necked round bottom flask and stirred at room temperature. 1-Ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC·HCl) (260 mg, 1.36 mmol), 1-hydroxybenzotriazole (HOBT) ( 184mg, 1.36mmol), N,N-diisopropylethylamine (DIPEA) (470mg, 3.636mmol), activated at room temperature for 1 hour. Finally, L-dimethylglutamic acid dimethyl hydrochloride (230 mg, 1.09 mmol) was added to the reaction system and stirred at room temperature for reaction. After 18 hours, the reaction was completed and the reaction was stopped. Add 10ml of water to the reaction system, adjust the pH to 5 with 1M dilute hydrochloric acid, extract 3 times with ethyl acetate (50ml×3), combine the organic phases and wash with saturated NaCl. Anhydrous Na for organic phase 2 S...
Embodiment 2
[0079] The synthesis of embodiment 2 compound DA-02
[0080]
[0081] The same operation was carried out in Example 1, only the L-glutamic acid methyl ester hydrochloride used in the example was changed to L-norleucine methyl ester hydrochloride to obtain a white solid powder with a yield of 73.5%.
[0082] DA-02 corresponds to the compound of formula I, where X represents NH, A1 represents norleucine, and A2 represents methyl.
[0083] 1 H NMR data are as follows: 1 H NMR (CDCl 3 ,300MHz)δ:0.87(t,3H,J=6.2Hz),1.25(s,2H),1.28(s,2H),1.67(s,1H),1.79(s,1H),2.82(s,1H ),2.97-3.05(m,1H),3.72(s,1H),4.25(s,1H),4.54(d,J=4.4Hz),5.30(s,1H),6.60(s,1H),6.76 (s,1H),7.16(d,1H,J=6.1Hz);
[0084] 13 The C NMR data are as follows: 13 C NMR (CDCl 3 ,75MHz) δ: 13.80, 22.21, 27.44, 31.81, 39.87, 52.13, 52.60, 72.73, 115.41, 116.42, 121.85, 128.40, 143.49, 144.02, 173.14.
Embodiment 3
[0085] The synthesis of embodiment 3 compound DA-03
[0086]
[0087] The same operation was carried out in Example 1, only the L-glutamic acid methyl ester hydrochloride used in the example was changed to L-tert-leucine methyl ester hydrochloride to obtain a white solid powder with a yield of 65.9%.
[0088] DA-03 corresponds to the compound of formula I, where X represents NH, A1 represents tert-leucine, and A2 represents methyl.
[0089] 1 H NMR data are as follows: . 1 H NMR (CDCl 3 ,300MHz)δ:0.96(s,9H),2.78-2.85(m,1H),2.97-3.03(m,1H),3.71(s,3H),4.26(dd,1H,J=4.0,7.3Hz) ,4.24(d,1H,J=9.3Hz),6.59(d,1H,J=7.8Hz),6,70(s,1H),6.77(d,1H,J=8.1Hz),7.19(d, 1H,J=9.2Hz);
[0090] 13 The C NMR data are as follows: 13 C NMR (CD 3 OD, 75MHz) δ: 25.40, 34.11, 39.58, 51.05, 59.86, 72.41, 114, 64, 116.43, 120.68, 128.48, 143.51, 144.50, 170.93, 174.55.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com