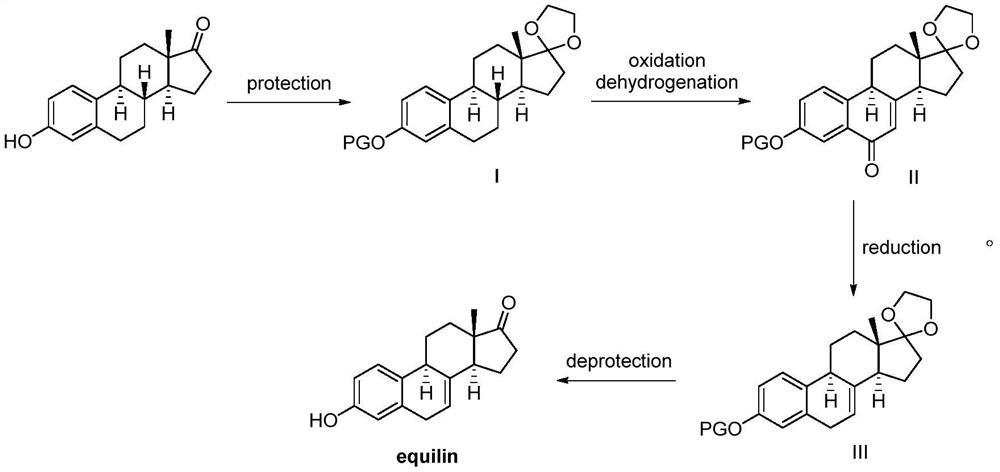
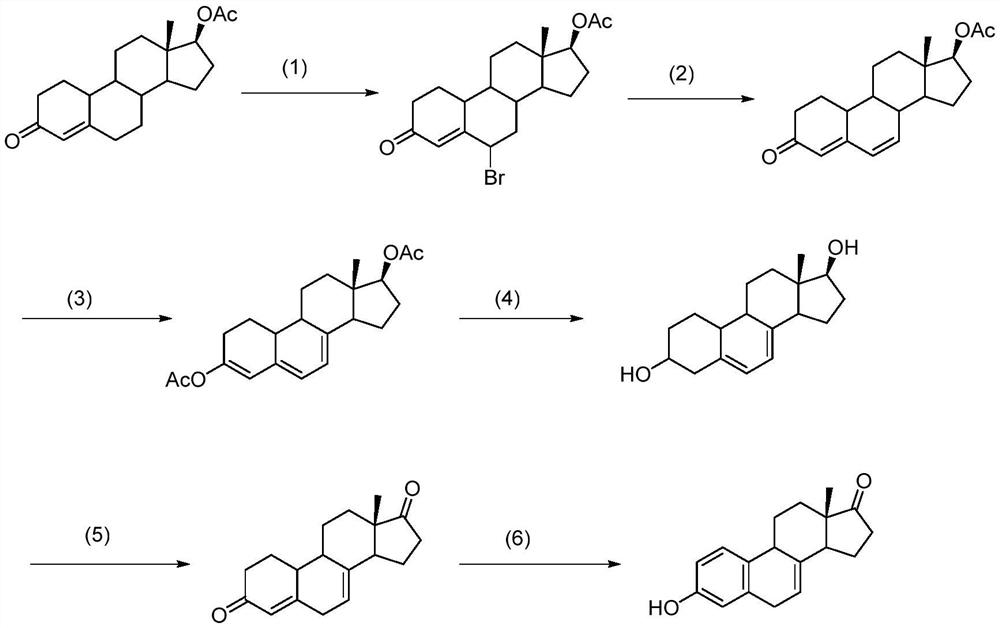
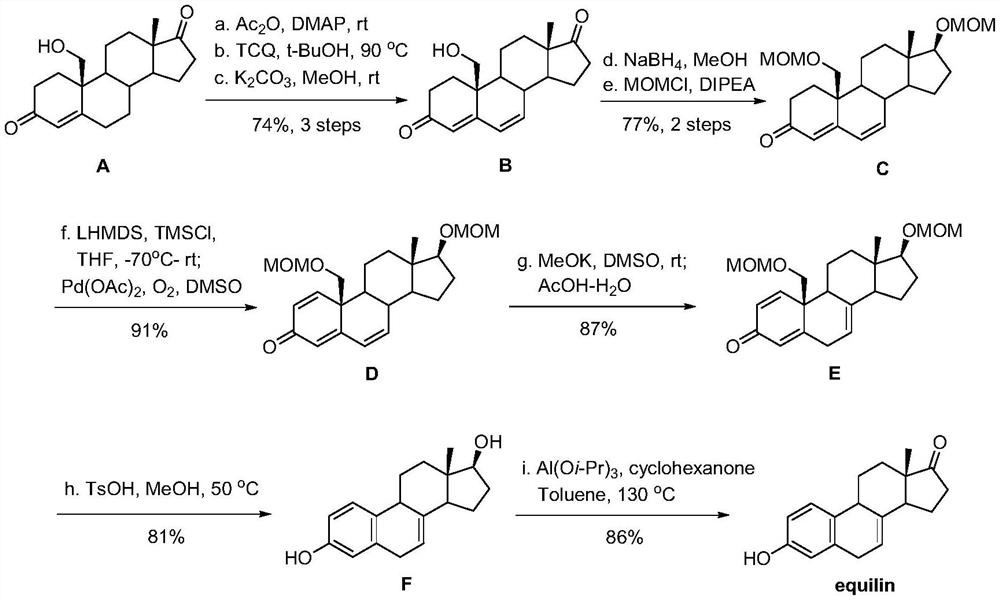
Method for synthesizing equilin
A technology for equine estrone and a synthesis method, which is applied in the field of synthesizing steroid drug equine estrone, can solve problems such as a large number of chemical oxidants, three waste discharges, environmental pollution and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1. Synthesis of Intermediate I (Acetyl protection at C3; ethylene glycol ketal protection at C17):
[0025] Acetic anhydride (150mmol, 15.3g) and 4-dimethylaminopyridine (3mmol, 0.367g) were successively added to estrone (30mmol, 8.1g), and the mixture was stirred at room temperature for 15 minutes. Thin layer chromatography showed that the raw material estrone had been converted. completely. After concentration, dichloromethane (20 mL) was added to dilute, and saturated NaHCO was added. 3 The solution to the system is neutral. After separation, the organic phase was washed with water, saturated brine, and anhydrous Na 2 SO 4 After drying, silica gel filtration and suction to dryness (solvent recovery) to obtain a white solid. The white solid was dissolved in anhydrous benzene (30 mL), then ethylene glycol (60 mmol, 3.72 g) and p-toluenesulfonic acid (3 mmol, 0.516 g) were added, heated to reflux for 5 hours, and cooled to room temperature. Saturated Na 2 ...
Embodiment 2
[0027] tert-butyryl, benzoyl, CBZ (benzyloxycarbonyl, O=C-OCH) 2 Ph), BOC (tert-butoxycarbonyl) and p-toluenesulfonyl anhydride instead of acetic anhydride, others are the same as in Example 1, and the yields of compound I are 75%, 70%, 78%, 72%, and 76%, respectively.
Embodiment 3
[0029] Respectively with acetyl, tert-butyryl, benzoyl, CBZ (benzyloxycarbonyl, O=C-OCH 2 Ph), BOC (tert-butoxycarbonyl) and p-toluenesulfonyl chloride instead of acetic anhydride, other are the same as in Example 1, the yields of compound I are 80%, 77%, 75%, 79%, 78%, respectively, 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com