One-pot On-DNA Suzuki reaction method
A Suzuki reaction and on-DNA technology, applied in the field of encoded compound libraries, can solve problems such as difficult separation and purification, inability to introduce DNA-encoded compound libraries, and limiting the diversity of DNA-encoded compound libraries.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] The synthesis of embodiment 1, 7 kinds of On-DNA aryl halide compounds
[0056]
[0057] DNA-NH 2 Dissolve in 250mM, pH=9.4 boric acid buffer solution, prepare 1mM concentration solution (20μL, 20nmol), acid aromatic halide (1μmol, 50 equivalent, 200mM DMA solution), HATU (1μmol, 50 equivalent, 400mM DMA solution), DIPEA (1 μmol, 50 equivalents, 400 mM DMA solution) was placed in a -20°C refrigerator for 5 minutes and then mixed. The mixture was thoroughly mixed by vortexing, and then placed in a 4°C refrigerator for 5 minutes; the above mixture Add to the solution of DNA raw materials, mix well, and leave overnight at room temperature.
[0058] Ethanol precipitation standard operating post-processing. Standard operation of ethanol precipitation: add a total volume of 10% 5M sodium chloride solution to the solution, and then continue to add 3 times the total volume of absolute ethanol. After shaking evenly, place the reaction in dry ice to freeze for 2 hours, and t...
Embodiment 2
[0059] Embodiment 2, the synthesis of On-DNA biaryl compound 4
[0060]
[0061] Dissolve B2pin2 in ACN to prepare a 1.2M concentration solution (0.11 μmol, 11 equivalents, 0.092 μL), and add BPO (0.002 μmol, 0.2 equivalents, 0.01 μL, 0.2M ACN solution), aromatic amine (0.1 μmol, 10 equivalents, 0.08 μL, 1.25M ACN solution), t-BuONO (0.15 μmol, 15 equivalents, 0.12 μL, 1.25M ACN solution), mixed in a 1.5mL centrifuge tube, shake evenly, and react at 25°C for 16 hours , for the next reaction.
[0062] The On-DNA aryl halogen raw material was dissolved in deionized water to prepare a 1mM concentration solution (10nmol, 1 equivalent, 10 μL), and the above mixed solution (100nmol, 10 equivalent, 0.302μL, 331mM ACN solution), KOH (1500 nmol, 150 equiv, 1.5 μL, 1M aqueous solution), POPd (6.7 nmol, 0.67 equiv, 0.335 μL, 20 mM DMA solution) and ligand (13.3 nmol, 1.33 equiv, 0.665 μL, 20 mM DMA solution) were mixed at 1.5 mL centrifuge tube, mix evenly, add to the aforementioned...
Embodiment 3
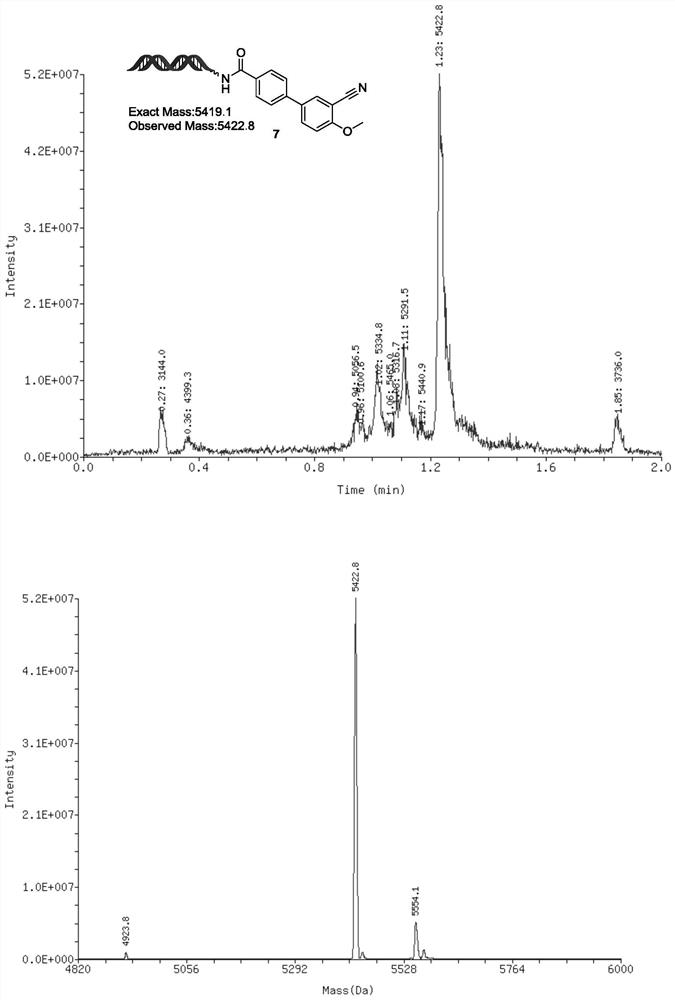
[0064] Embodiment 3, the synthesis of On-DNA biaryl compound 7
[0065]
[0066] Dissolve B2pin2 in ACN to prepare a 1.2M concentration solution (0.11 μmol, 11 equivalents, 0.092 μL), and add BPO (0.002 μmol, 0.2 equivalents, 0.01 μL, 0.2M ACN solution), aromatic amine (0.1 μmol, 10 equivalents, 0.08 μL, 1.25M ACN solution), t-BuONO (0.15 μmol, 15 equivalents, 0.12 μL, 1.25M ACN solution), mixed in a 1.5mL centrifuge tube, shake evenly, and react at 25°C for 16 hours , for the next reaction.
[0067] Dissolve the On-DNA aryl halogen raw material in deionized water to prepare a 1mM concentration solution (10nmol, 1 equivalent, 10μL), and add the above mixed solution (100nmol, 10 equivalent, 0.302μL, 331mM ACN solution), KOH (1500nmol, 150 equivalents, 1.5μL, 1M aqueous solution), POPd (6.7nmol, 0.67 equivalents, 0.335μL, 20mM DMA solution) and ligand (13.3nmol, 1.33 equivalents, 0.665μL, 20mM DMA solution) were mixed in 1.5mL In a centrifuge tube, mix evenly, add to the af...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com