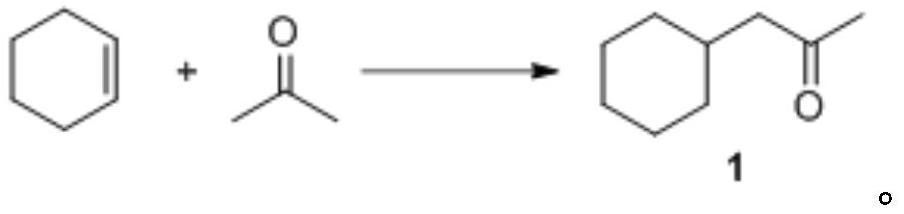
Preparation method of cyclohexyl acetone
A technology of cyclohexylacetone and acetone, which is applied in the field of preparation of cyclohexylacetone, can solve problems such as inability to carry out large-scale production, and achieve the effects of increased yield, simple post-treatment, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] To acetone (580.80 g, 10 mol) in acetic acid (1 L) was added manganese acetate (4.90 g, 0.02 mol), cobalt acetate (0.89 g, 5 mmol) and cyclohexene (82.15 g, 1 mol) in sequence. The temperature was raised to 65°C, and oxygen was introduced into the reaction liquid. After the gas phase detection reaction was completed, it was lowered to room temperature. The insoluble matter was removed by filtration, and the filtrate was distilled under reduced pressure to obtain the product (85.54 g, 61%). The evaporated acetone and acetic acid can be recycled and reused. 1 H NMR (CDCl 3 , 400MHz) δ2.28(d, 2H, J=6.8Hz); 2.11(s, 3H); 1.85-1.75(m, 1H); 1.69-1.61(m, 4H); 1.30-1.08(m, 4H) ; 0.94-0.85 (m, 2H).
Embodiment 2
[0044] To a solution of acetone (58.08 g, 1 mol) in acetic acid (100 mL), nickel acetylacetonate (0.26 g, 1 mmol), cobalt acetate (0.09 g, 0.5 mmol) and cyclohexene (8.22 g, 0.1 mol) were added sequentially. The temperature was raised to 65°C, and oxygen was introduced into the reaction liquid. After the gas phase detection reaction was completed, the product (5.05 g, 36%) was obtained according to the post-treatment method of Example 1.
Embodiment 3
[0046] To acetone (58.08 g, 1 mol) in acetic acid (100 mL) was added manganese acetate (0.49 g, 2 mmol), cobalt acetate (0.09 g, 0.5 mmol) and cyclohexene (8.22 g, 0.1 mol) in sequence. The temperature was raised to 65° C., and air was introduced into the reaction liquid. After the gas phase detection reaction was completed, the product (7.43g, 53%) was obtained according to the post-processing method of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com