Preparation method of 2-amino-4-nitrophenol
A technology of nitrophenol and aminophenol is applied in the field of preparation of 2-amino-4-nitrophenol, can solve the problems of unsuitability for large-scale production and use, high price, low selectivity, etc., achieves good industrialization prospects, and is simple to operate , good appearance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
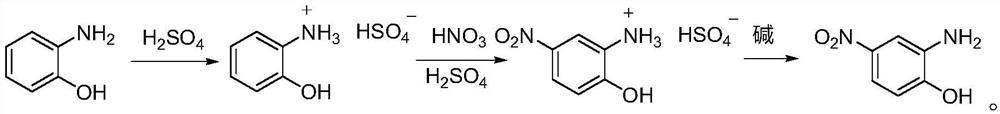
[0038] The invention provides a kind of preparation method of 2-amino-4-nitrophenol, comprising the following steps:
[0039] (1) Synthesis of 2-aminophenol bisulfate:
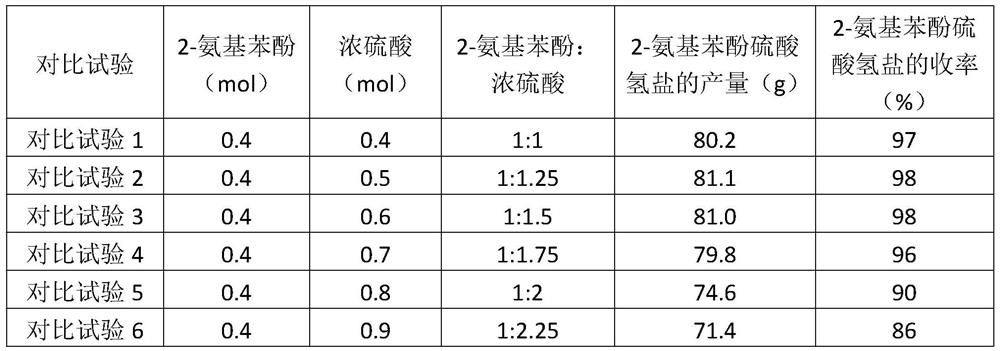
[0040] 2-Aminophenol (0.4mol, 43.7g) and 200g of toluene were added to a 250mL three-necked flask, and under nitrogen protection, 98% concentrated sulfuric acid (0.5mol, 50.0g) was slowly added dropwise, and the reaction was stirred for 3 hours to form 2-Aminophenol bisulfate, cooled to 25°C after the reaction, suction filtered and vacuum dried to obtain 81.1 g of 2-aminophenol bisulfate, with a yield of 98%;
[0041] (2) Synthesis of 2-amino-4-nitrophenol crude product:
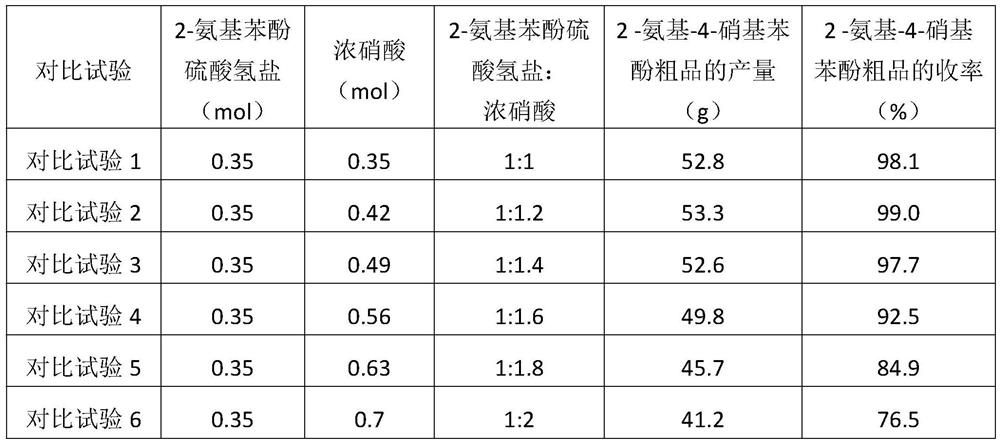
[0042] The 2-aminophenol bisulfate (0.35mol, 72.5g) prepared in step (1) and 200g toluene were added in a 250mL three-necked flask, and under nitrogen protection, mixed acid (28ml of 98% concentrated sulfuric acid and 68 % concentrated nitric acid 28ml, the molar number of concentrated nitric acid is 0.42mol), after 1h dripping, heat up to...
Embodiment 2
[0046] The invention provides a kind of preparation method of 2-amino-4-nitrophenol, comprising the following steps:
[0047] (1) Synthesis of 2-aminophenol bisulfate:
[0048] 2-Aminophenol (0.4mol, 43.7g) and 200g of dichloroethane were added to a 250mL three-necked flask, and under nitrogen protection, 98% concentrated sulfuric acid (0.5mol, 50.0g) was slowly added dropwise, and the stirring reaction 2 After 2 hours, 2-aminophenol hydrogensulfate was generated, and after the reaction, it was cooled to 30° C., and after suction filtration and vacuum drying, 80.3 g of 2-aminophenol hydrogensulfate was obtained, with a yield of 97%;
[0049] (2) Synthesis of 2-amino-4-nitrophenol crude product:
[0050]The 2-aminophenol bisulfate (0.35mol, 72.5g) prepared in step (1) and 200g ethylene dichloride were added to a 250mL three-necked flask, and mixed acid (98% concentrated sulfuric acid) was added dropwise under nitrogen protection. 28ml and 68% concentrated nitric acid (28ml, t...
Embodiment 3
[0054] The invention provides a kind of preparation method of 2-amino-4-nitrophenol, comprising the following steps:
[0055] (1) Synthesis of 2-aminophenol bisulfate:
[0056] 2-Aminophenol (0.4mol, 43.7g) and 200g 2-methyltetrahydrofuran were added to a 250mL three-necked flask, and under nitrogen protection, 98% concentrated sulfuric acid (0.5mol, 50.0g) was slowly added dropwise, and the reaction was stirred After 2 hours, 2-aminophenol hydrogensulfate was generated. After the reaction, it was cooled to 25° C., and 78.6 g of 2-aminophenol hydrogensulfate was obtained after suction filtration and vacuum drying, with a yield of 95%.
[0057] (2) Synthesis of 2-amino-4-nitrophenol crude product:
[0058] The 2-aminophenol bisulfate (0.35mol, 72.5g) prepared in step (1) and 200g 2-methyltetrahydrofuran were added to a 250mL three-necked flask, and mixed acid (98% concentrated Sulfuric acid 28ml and 68% concentrated nitric acid 28ml, the molar number of concentrated nitric ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com