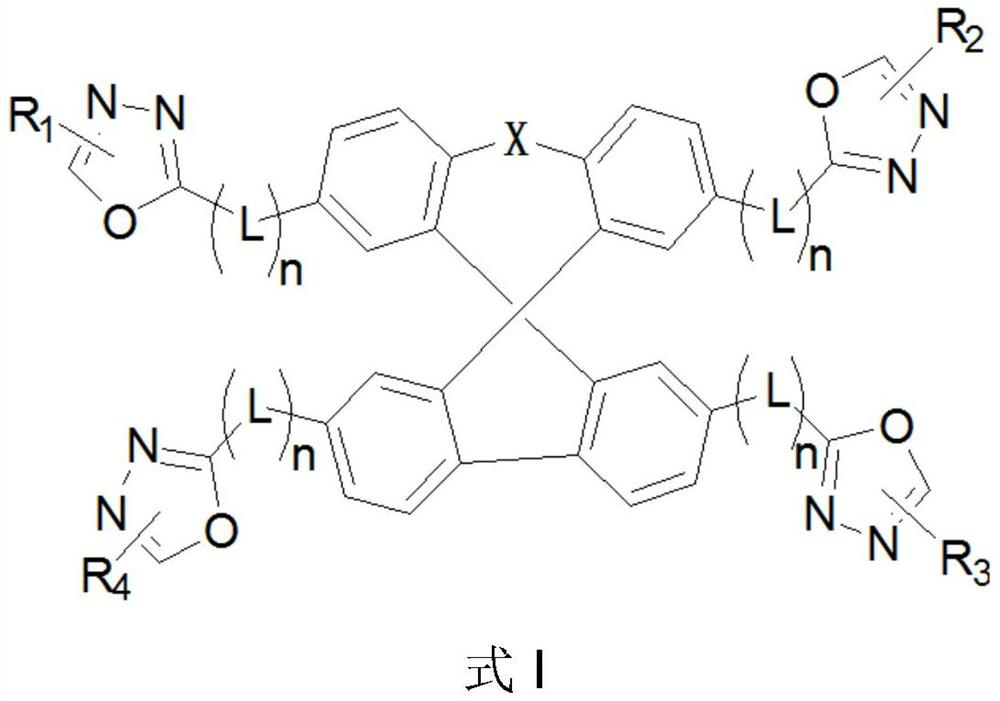
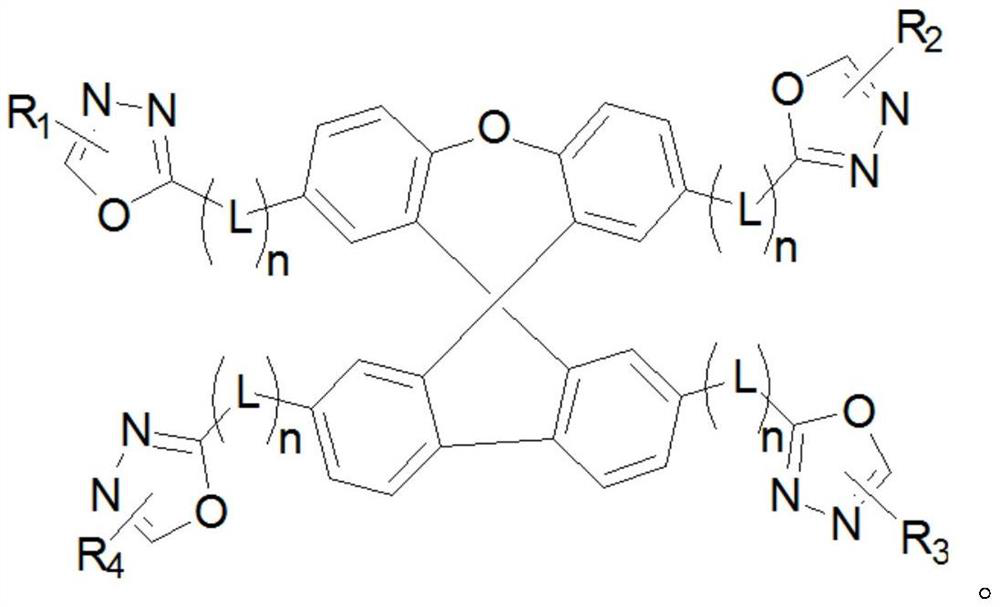
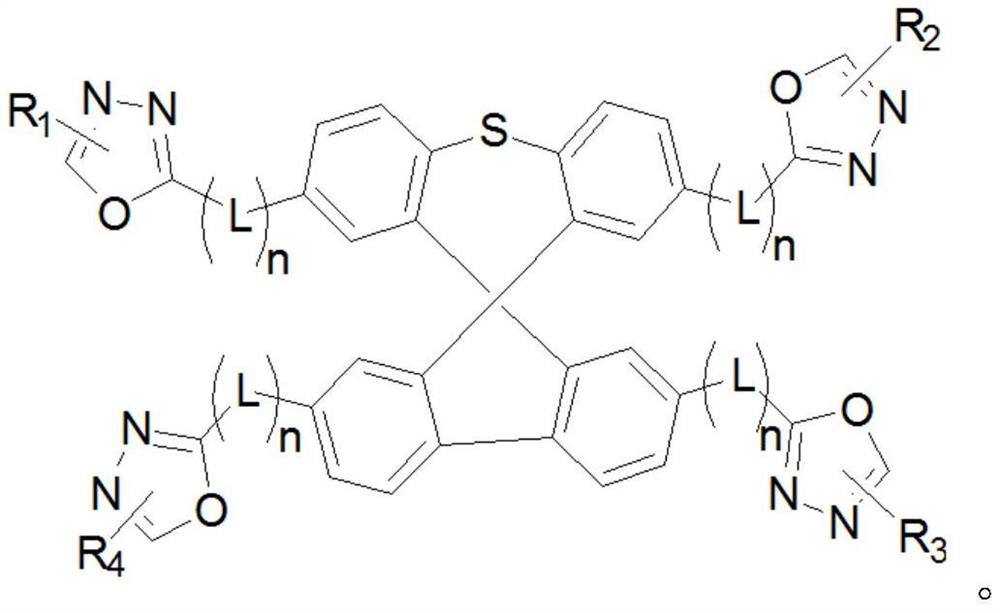
Oxadiazole-substituted organic light-emitting material and OLED device
A light-emitting material, oxadiazole technology, applied in the field of OLED devices, can solve the problems of shortened life, increased driving voltage, decreased luminous efficiency, etc., and achieves the effect of high glass transition temperature, high charge transfer ability, and prevention of crystallization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]Preparation equation is:
[0035]
[0036]Compound 1
[0037]Step is:
[0038]5g of 1,8 g of anhydrous phosphate powder, 100ml no water 1,4-dioxane and 0.6 g of Pd (PPH)3)4In the 200 ml of three bottles, the nitrogen gas was drifted for 30 min. To maintain 101 ° C, 8.5 g of 1,3,4-malodolic acid was added, and the lateral reflux reaction was taken 24 h. After cooling to room temperature, the solid, filtered, was filtered, and the column chromatography was precipitated to give 2.3 g of the product (yield 46%).
[0039]The final product was analyzed: Tg (DSC) 110 ° C, purity 99.9%,1H NMR (400 MHz, DMSO) δ 7.78 (S, 4H), 7.66 (S, 4H), 7.40 (D, 4H), 7.04 (D, 4H).
Embodiment 2
[0041]Preparation equation is:
[0042]
[0043]Compound 2
[0044]Step is:
[0045]5 g of 5 g of compound 2,8 g of potassium powder, 100ml no water 1,4-dioxane and 0.6 g of Pd (PPH)3)4In the 200 ml of three bottles, the nitrogen gas was drifted for 30 min. To maintain 101 ° C, 8.5 g of 1,3,4-malodolic acid was added, and the lateral reflux reaction was taken 24 h. After cooling to room temperature, the solid, filtered, chromatography was precipitated, and 2.2 g of the product (yield 42%) was obtained.
[0046]The final product was analyzed: Tg (DSC) 113 ° C, purity 99.9%,1H NMR (400 MHz, DMSO) δ 7.78 (S, 4H), 7.76 (S, 2H), 7.60 (D, 2H), 7.50 (M, 4H), 7.32 (D, 2H), 7.24 (D, 2H) .
Embodiment 3
[0048]Preparation equation is:
[0049]
[0050]Compound 3
[0051]Step is:
[0052]5 g of 5 g of compound 3,8 g of potassium powder, 100 ml no water 1,4-dioxane and 0.6 g of Pd (PPH)3)4In the 200 ml of three bottles, the nitrogen gas was drifted for 30 min. To maintain 101 ° C, 8.5 g of 1,3,4-malodolic acid was added, and the lateral reflux reaction was taken 24 h. After cooling to room temperature, solid, filtrate, chromatography (41% yield) was obtained.
[0053]The final product was analyzed:
[0054]Tg (DSC) 115 ° C, purity 99.9%,1H NMR (400 MHz, DMSO) δ 7.76 (S, 2H), 7.60 (D, 2H), 7.50 (D, 4H), 7.48 (D, 8H), 7.32 (M, 10H), 7.24 (D, 2H) , 7.22 (m, 4h).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com