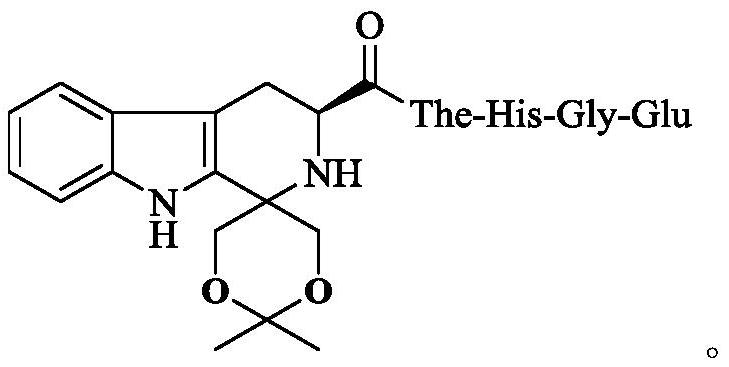
Dioxane-modified tetrahydrocarboline-3-formyl-The-HGE as well as preparation, antithrombotic activity and application thereof
A technology of dioxane and carboline, applied in the preparation of anti-arterial thrombosis drugs, in the field of arterial thrombosis activity, can solve problems such as no substantial progress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
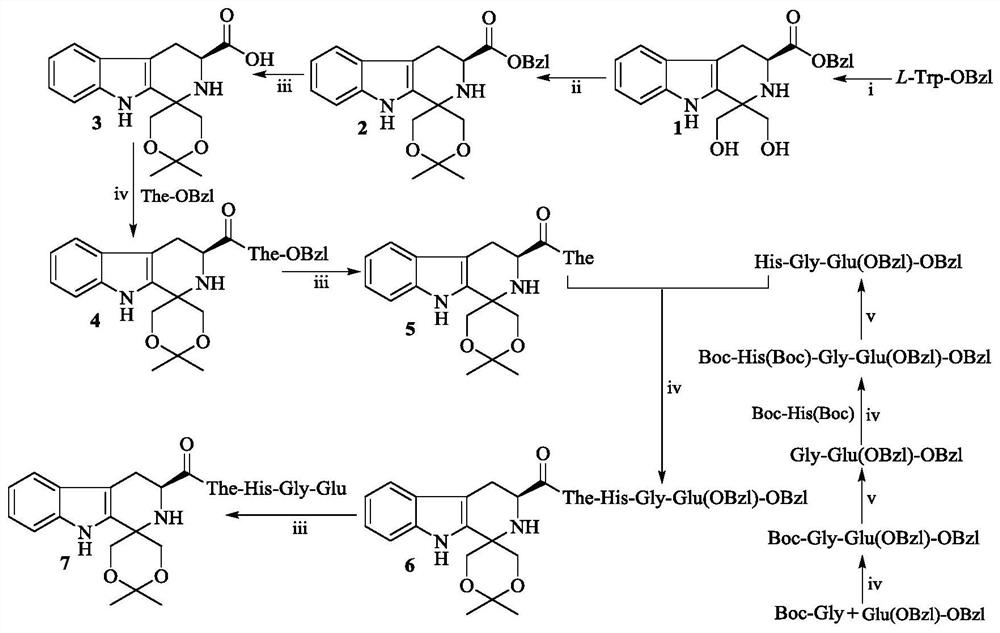
[0021] Example 1 Preparation of (3S)-1,1-dimethylol-tetrahydro-β-carboline-3-carboxylic acid benzyl ester (1)
[0022] Add 2g (7.2mmol) L-tryptophan benzyl ester to 10mL of dichloromethane, stir well to dissolve it. Under the condition of ice bath, slowly drop 1mL trifluoroacetic acid into the solution. Then add 0.78g (8.6mmol) 1,3-dihydroxyacetone to the solution, and react at room temperature for 7 hours. TLC showed the disappearance of L-tryptophan benzyl ester (dichloromethane / methanol: 30:1). Under ice bath conditions, add 50mL saturated NaHCO to the solution 3 solution, stirred well, then left the dichloromethane layer, and then washed with saturated NaHCO 3 solution (30mL×3), washed with saturated NaCl solution (30mL×3), and the dichloromethane layer was dried over anhydrous sodium sulfate for 12 hours. Filtration and concentration of the filtrate under reduced pressure afforded 2.03 g (77%) of the title compound as a yellow powder. ESI-MS(m / e):367[M+H] + .
Embodiment 2
[0023] Example 2 Preparation of 3S-1-(1,1-dimethyl-1,3-dioxane-6-spiroyl)-1,2,3,4-tetrahydro-β-carboline-3- Benzyl carboxylate (2)
[0024] Add 0.2 g (0.55 mmol) (3S)-1,1-dimethylol-tetrahydro-β-carboline-3-carboxylic acid benzyl ester (1) to 5 mL of anhydrous acetone. Under ice bath conditions, 100 μL of concentrated sulfuric acid was added thereto, and then stirred at room temperature for 3 hours. TLC showed that compound 1 disappeared (petroleum ether / ethyl acetate, 4:1). In an ice bath, with saturated NaHCO 3 The pH value of the reaction solution was adjusted to 7, the obtained solution was concentrated under reduced pressure to remove acetone, the residual solution was extracted and washed with ethyl acetate three times, and the ethyl acetate layer was washed with saturated NaCl solution until neutral. The ethyl acetate layer was dried over anhydrous sodium sulfate for 12 hours. After filtration, the filtrate was concentrated under reduced pressure to obtain a tan sol...
Embodiment 3
[0025] Example 3 Preparation of 3S-1-(1,1-dimethyl-1,3-dioxane-6-spiroyl)-1,2,3,4-tetrahydro-β-carboline-3- Carboxylic acid (3)
[0026] Add 0.20g (0.5mmol) 3S-1-(1,1-dimethyl-1,3-dioxane-6-spiroyl)-1,2,3,4-tetrahydro- β-carboline-3-carboxylate benzyl ester (2) and 0.02 g Pd / C. Stir and pass hydrogen for 12h, TLC showed that compound 2 disappeared (petroleum ether / ethyl acetate, 4:1). Palladium on carbon (Pd / C) was removed by filtration, and the filtrate was concentrated under reduced pressure. The residue was triturated with ether to afford 0.14 g (90%) of the title compound as a colorless solid. ESI-MS(m / e):317[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com