Preparation method of tofacitinib impurity
A technology of tofacitinib and impurities, which is applied in the fields of drug synthesis and drug quality research, can solve the problems of low compound yield, severe reaction conditions, and difficult to control, and achieves high yield, simple process, and easy separation and purification. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
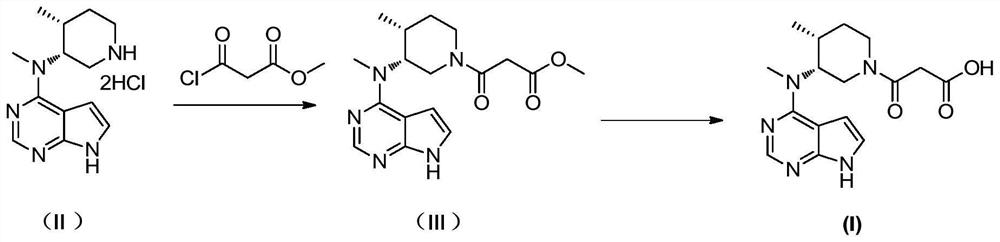
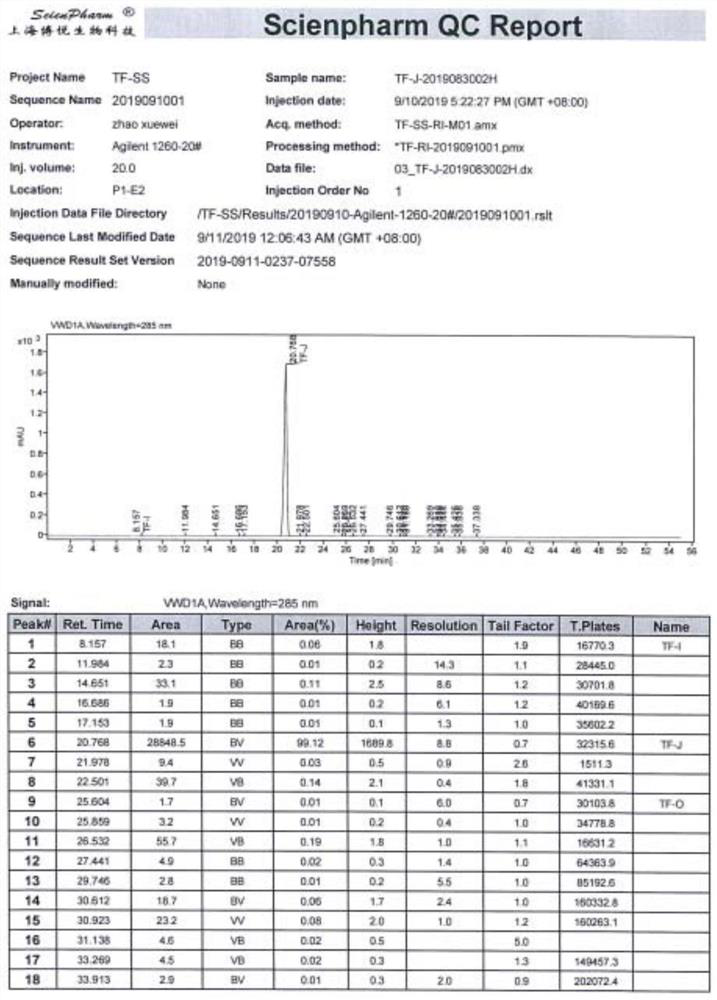
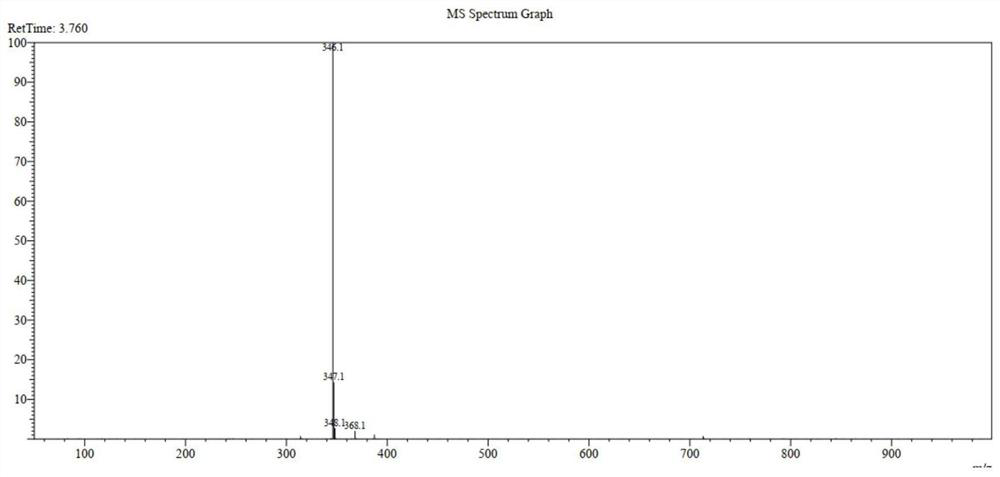
[0058] Tofacitinib impurity 3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidin-1-yl )-3-oxopropionic acid preparation. The synthesis process is as figure 1 shown; the specific steps are as follows:
[0059] A, the preparation of the compound shown in formula (Ⅲ);
[0060] Add 9.5 g (29.8 mmol) of the compound represented by formula (II) into 95 mL of dichloromethane, add 12.1 g (119.4 mmol) of triethylamine, and stir. The temperature was lowered to 0-5° C., and a solution of 6.5 g (47.8 mmol) of methyl 3-chloro-3-oxopropionate in dichloromethane (10 mL) was added dropwise. After the dropwise addition, the temperature was naturally raised to 20-30°C. After 3 hours of reaction, the temperature was lowered to 0-5°C, and 5mL of water was added to quench the reaction, and 2N dilute hydrochloric acid was added dropwise to adjust the pH value to 5-6. The liquid was separated, and the organic phase was sequentially Wash with saturated sodium bicarbonate ...
Embodiment 2
[0068] Tofacitinib impurity 3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidin-1-yl )-3-oxopropionic acid preparation
[0069] A, the preparation of the compound shown in formula (Ⅲ);
[0070] Add 1 g (3.1 mmol) of the compound represented by formula (II) into 5 mL of dichloromethane, add 1.11 g (11.0 mmol) of triethylamine, and stir. The temperature was lowered to 0-5° C., and a solution of 0.5 g (3.8 mmol) of methyl 3-chloro-3-oxopropionate in dichloromethane (2 mL) was added dropwise. After the dropwise addition, naturally raise the temperature to 20-30°C, after 2-3 hours of reaction, cool down to 0-5°C, add 2mL water to quench the reaction, add dilute hydrochloric acid dropwise to adjust the pH value to 5-6, separate liquid, organic phase Washed with saturated sodium bicarbonate and saturated brine, dried the organic phase with an appropriate amount of anhydrous sodium sulfate, filtered, concentrated to dryness under reduced pressure, and purif...
Embodiment 3
[0074] Tofacitinib impurity 3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidin-1-yl )-3-oxopropionic acid preparation
[0075] A, the preparation of the compound shown in formula (Ⅲ);
[0076] Add 1 g (3.1 mmol) of the compound represented by formula (II) into 20 mL of dichloromethane, add 2.22 g (22.0 mmol) of triethylamine, and stir. The temperature was lowered to 0-5°C, and a solution of 857 mg (6.3 mmol) of methyl 3-chloro-3-oxopropionate in dichloromethane (3 mL) was added dropwise. After the dropwise addition, the temperature was naturally raised to 20-30°C. After 3 hours of reaction, the temperature was lowered to 0-5°C, and 2 mL of water was added to quench the reaction. Dilute hydrochloric acid was added dropwise to adjust the pH value to 5-6. Wash with saturated sodium bicarbonate and saturated brine, dry the organic phase with an appropriate amount of anhydrous sodium sulfate, filter, concentrate to dryness under reduced pressure, and pu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com