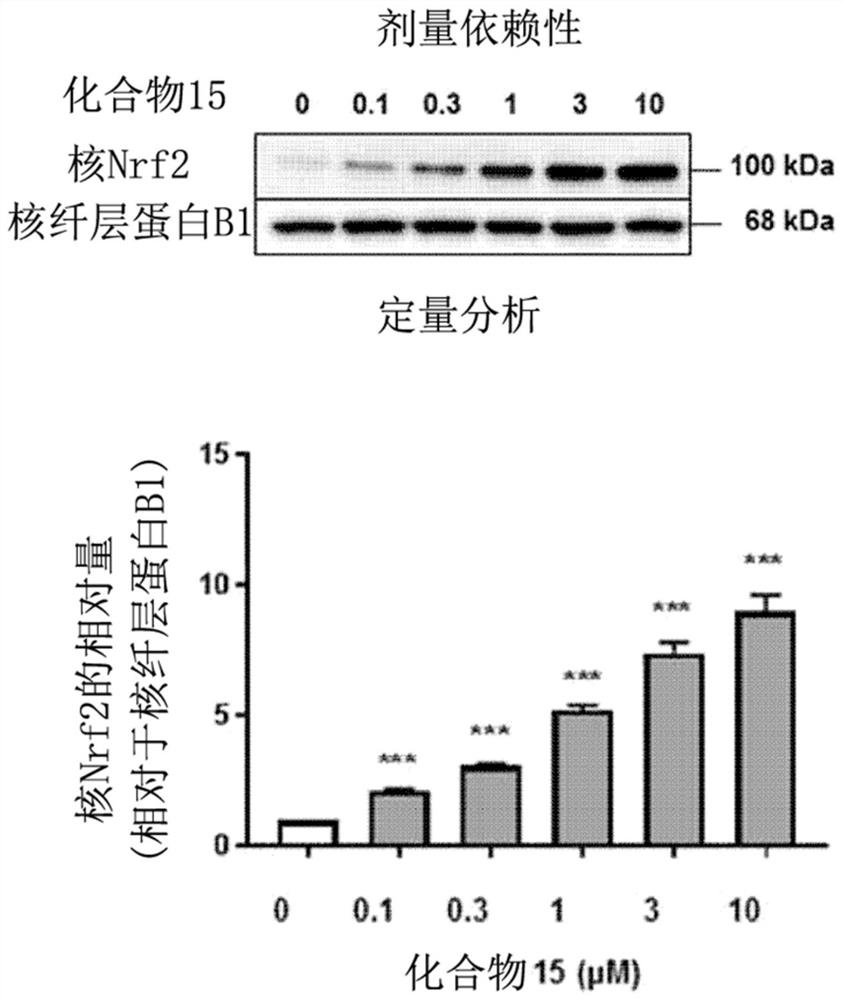
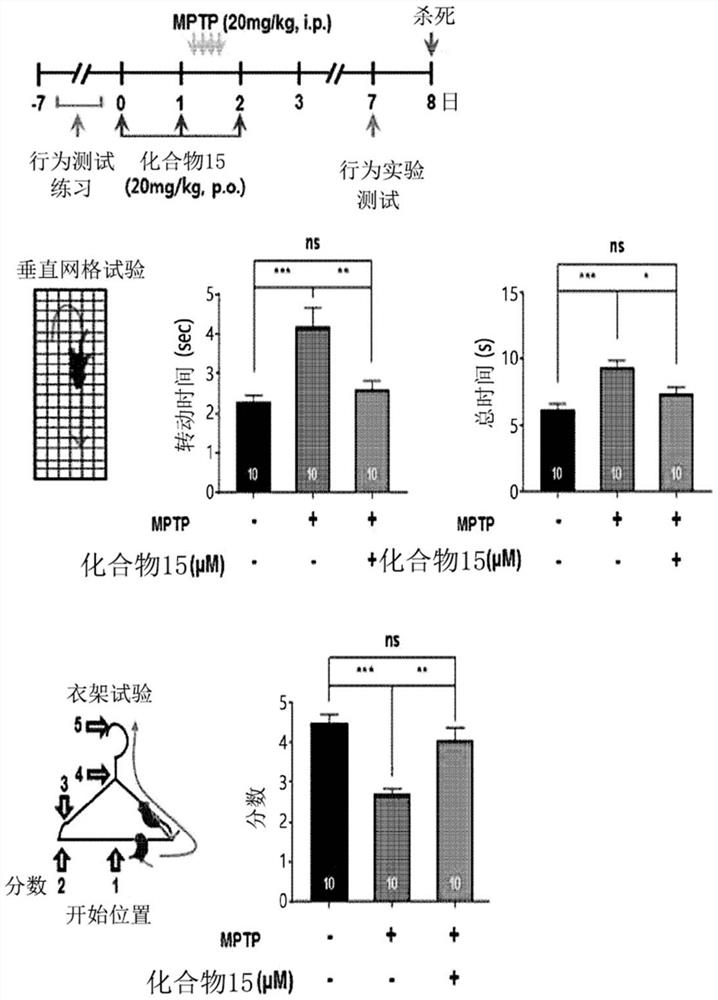
Novel halo-(3-(phenylsulfonyl)prop-1-enyl)pyridine derivative and use thereof
A chlorobenzenesulfonyl and fluorobenzenesulfonyl technology, applied in the field of Nrf2 activators, can solve the problems of low solubility, bioavailability and drug metabolism stability, and achieve the effect of treating or preventing diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0067] The preparation method of the present invention may further include reacting with an excess acidic solution to provide the compound represented by Chemical Formula 1 in a salt form after the reaction, but is not limited thereto.
[0068] For example, in the preparation method of the present invention, when R 1 for , used as a reactant ((R 1 -substituted phenyl) sulfonyl) methyl phosphonic acid diethyl ester can be obtained by making (hydroxyl-C 1-5 Alkyl) morpholines react with methanesulfonyl halides to prepare morpholinyl (C 1-5 Alkyl) methanesulfonate the first a-1 step; And make morpholino (C 1–5 Preparation for step a-2 of reaction of alkyl)methanesulfonate with diethyl (hydroxyphenylsulfonyl)methylphosphonate, but not limited thereto. For example, commercially available products can be purchased and used, or products can be used without limitation and / or synthesized using modified methods known in the art.
[0069] Here, the a-1th step can be obtained by add...
preparation example 1
[0097] Preparation Example 1: Synthesis of (hydroxybenzenesulfonyl) methylphosphonic acid diethyl ester
[0098] Step 1-1: Synthesis of methyl (diethoxyphosphoryl)4-methylbenzenesulfonate from diethyl hydroxymethylphosphonate
[0099]
[0100] (diethoxyphosphoryl)methyl4-methylbenzenesulfonate was synthesized according to the above reaction formula. Specifically, diethyl hydroxymethylphosphonate (diethyl hydroxymethylphosphonate, 10 g, 0.06 mol) was dissolved in methylene chloride (MC), and triethylamine ((triethylamine, 9.80 mL, 0.07 mmol) and 4 - Toluenesulfonyl chloride (4-toluenesulfonyl chloride, 13.3g, 0.07mol), stirred at room temperature for 3.5 hours. After the reaction was completed, dilute the reaction solution with ethyl acetate (ethyl acetate; EtOAc), wash with water and brine (brine), and then wash with Anhydrous Na 2 SO 4 The organic layer was dried and filtered. The residue obtained by distillation of the solvent under reduced pressure was purified by ...
Embodiment 1
[0197] Example 1: Synthesis of (E)-3-fluoro-2-(2-(2-fluorobenzenesulfonyl)vinyl)pyridine (compound 1)
[0198]
[0199] After dissolving diethyl (2-fluorobenzenesulfonyl)methylphosphonate (1-3a, 1 eq) prepared according to Preparation 1 in anhydrous THF (0.1 M), the reaction mixture was distilled using dry ice and acetone Cool to -78°C. n-BuLi (1.2eq, 2.0M solution in cyclohexane) was slowly dropped into the solution and stirred for 1 hour, then 3-fluoropicolinaldehyde (3-fluoropicolinaldehyde, 1.2eq) was added and reacted for another hour. If the reaction was confirmed to be incomplete by TLC, the reaction was carried out at room temperature for another 30 minutes. After quenching the reaction with a small amount of water, the reaction mixture was extracted with water and 10% MeOH / MC, washed with anhydrous Na 2 SO 4 A small amount of water was removed from the organic layer, and the solvent was distilled off under reduced pressure and dried in vacuo. Then, the product ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com