Synthesis method of indanone derivatives
A synthesis method and derivative technology, applied in the field of synthesis of indanone derivatives, to achieve the effects of high reaction yield, simple operation, and good economy of atoms and steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~9
[0055] Add benzoic acid compound (0.3mmol), acrylate compound (0.6mmol), pentamethylcyclopentadiene rhodium dichloride (0.015mmol), sodium acetate (0.3mmol) in test tube according to the raw material ratio of Table 1 ) and organic solvent 0.4ml, mixed and stirred evenly, after the reaction was completed according to the reaction conditions in Table 2, cooling, suction filtration, silica gel mixing sample, and obtaining the corresponding 2-substituted indanone compound (I) through column chromatography purification;
[0056] The reaction process is shown in the following formula:
[0057]
[0058] The raw material ratio of table 1 embodiment 1~9
[0059]
[0060] The reaction conditions and reaction result of table 2 embodiment 1~9
[0061]
[0062]
[0063] In Table 2, T is the reaction temperature, and t is the reaction time.
Embodiment 10
[0065] Add 2-substituted indanone derivative (I-1) (0.3mmol), sodium acetate (0.3mmol), and hexafluoroisopropanol (HFIP) 0.4ml into the test tube, mix and stir evenly under nitrogen, place in React in an oil bath at 150°C for 18 hours. After completion of the reaction, cooling, suction filtration, sample mixing with silica gel, purification by column chromatography to obtain the corresponding non-substituted indanone compound (I-10), the reaction process is shown in the following formula:
[0066]
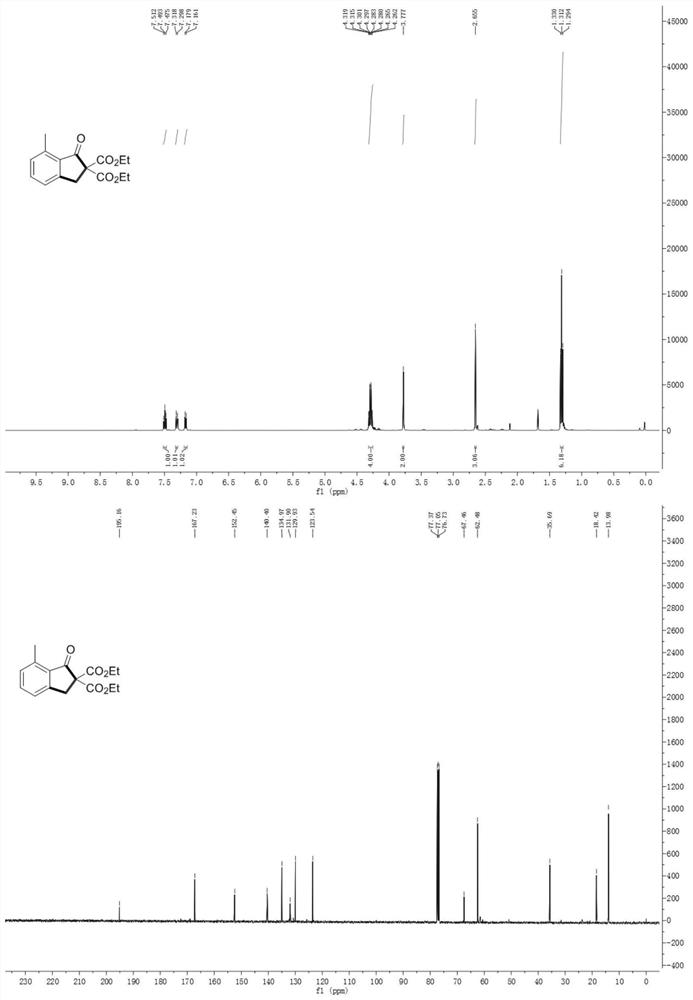
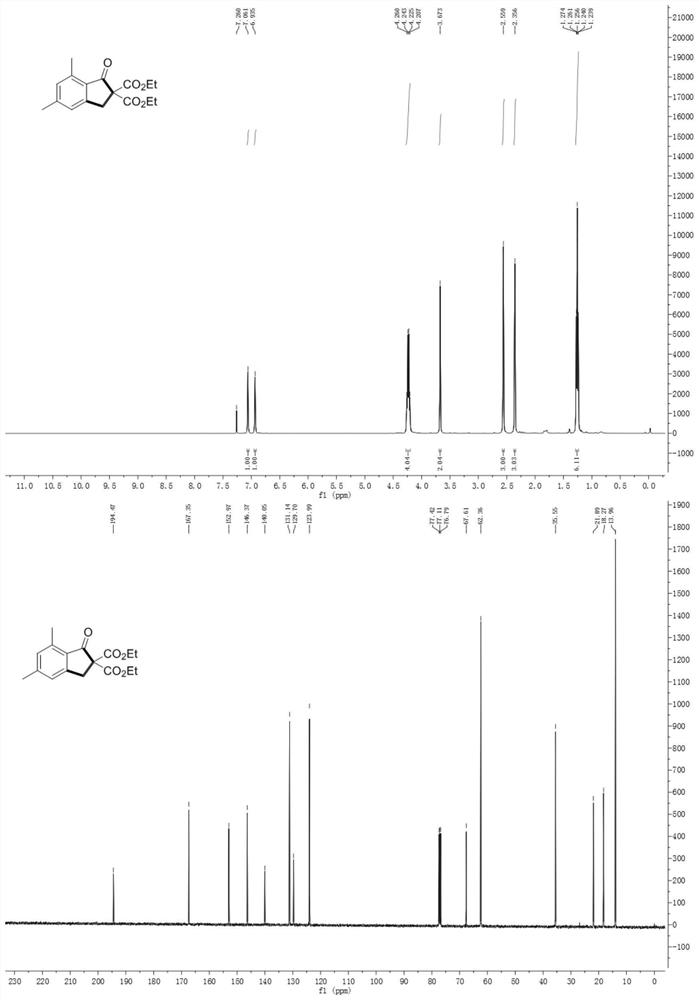
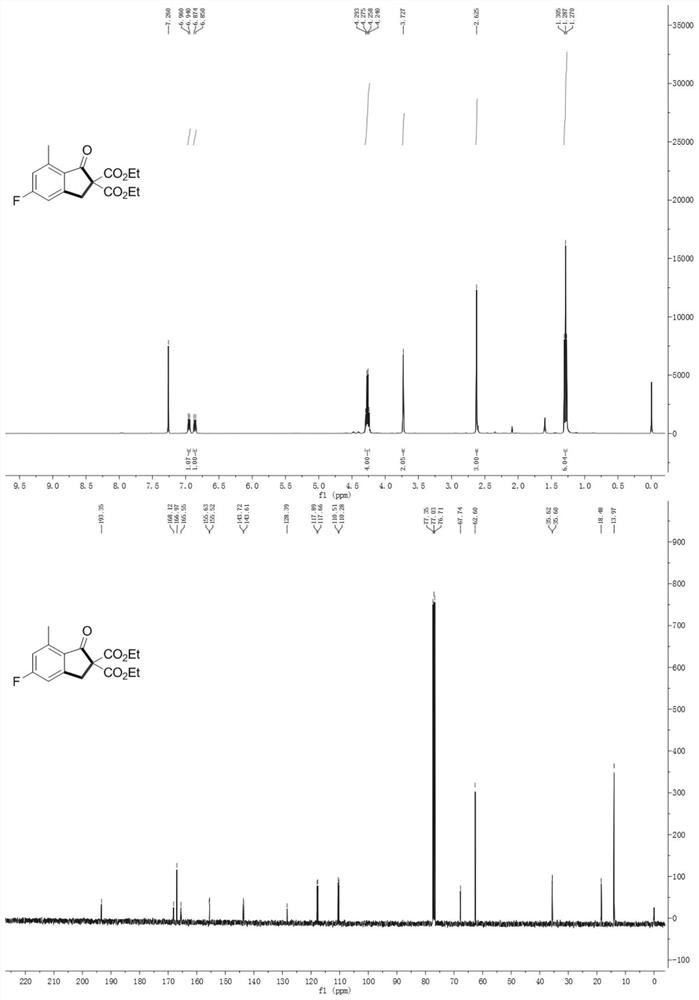
[0067] The data characterization of indanone compound (I) is as follows: Figure 1-10 As shown, wherein, the hydrogen spectrum is tested on a 400MHz nuclear magnetic instrument; the carbon spectrum is tested on a 100MHz nuclear magnetic instrument; the test conditions are all using tetramethylsilane as an internal standard at room temperature, and the sample is dissolved in deuterated chloroform.
[0068] The structures and relevant data of some compounds prepared in Examples 1-1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com