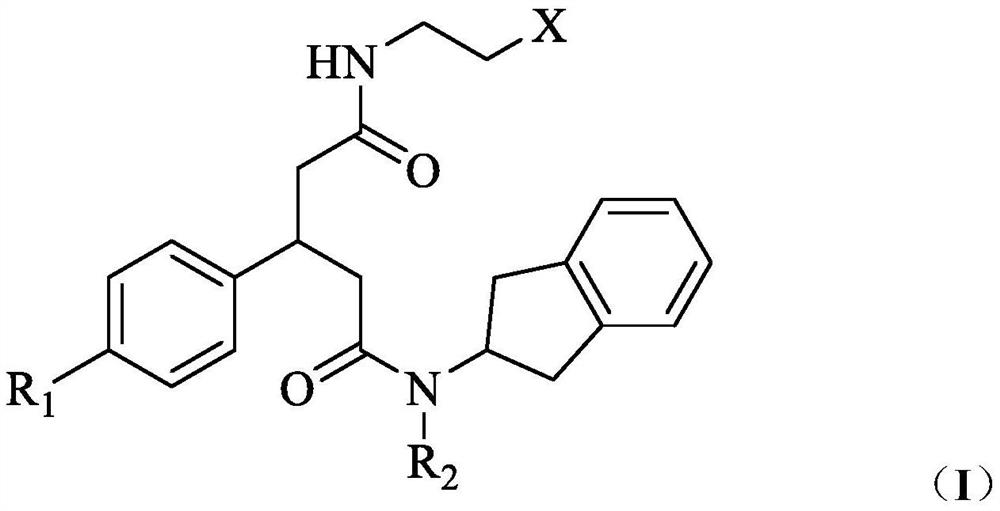
Glycinamide derivative as well as preparation method and application thereof
A technique for glycinamide and derivatives, applied in the field of glycinamide derivatives and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
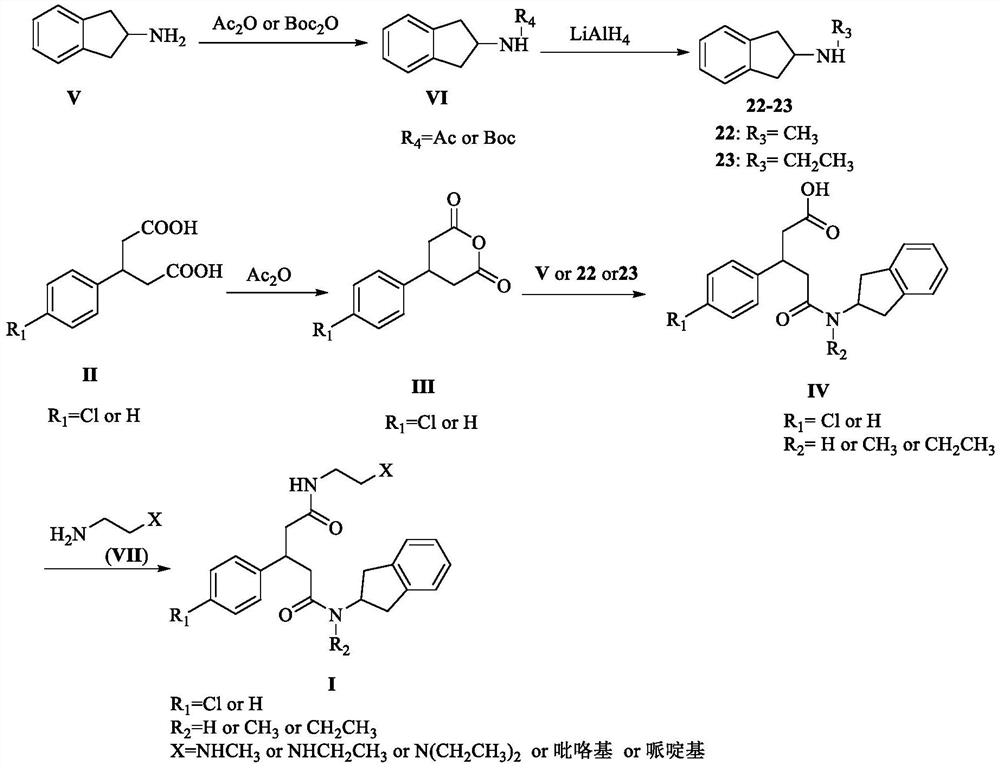
[0031] Embodiment 1: the preparation of compound 22-23
[0032]
[0033] Compound 2,3-dihydro-1H-indan-2-amine hydrochloride (V, 0.012mol), triethylamine (0.035mol), di-tert-butyl dicarbonate (0.013mol) were dissolved in dichloromethane (50mL), stirred at room temperature for 3h, after the reaction was complete, added water (20mL×2) to wash, washed once with saturated brine, anhydrous MgSO 4 Dry, filter, and concentrate to give a white solid intermediate;
[0034] In the reaction flask, first add LiAlH 4 (0.024mol), under nitrogen protection, slowly add anhydrous tetrahydrofuran (25mL) under ice bath, then dissolve the white solid intermediate in anhydrous tetrahydrofuran (35mL), and slowly drop into the reaction flask under ice bath ;
[0035] After dropping, heat to reflux, after the reaction is complete, cool to room temperature, pour the reaction solution into 10% aqueous sodium hydroxide solution (120mL), then extract with dichloromethane (60mL×3), wash once with sa...
Embodiment 2
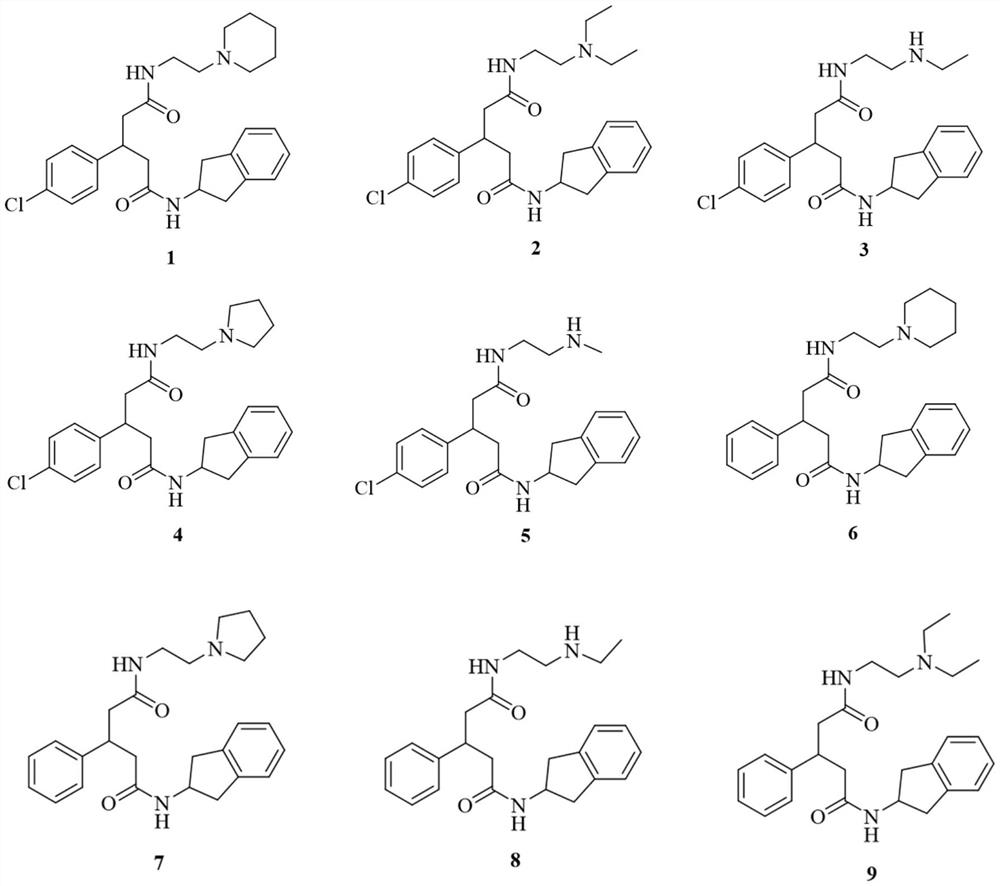
[0041] Embodiment 2: the preparation of compound 1-5
[0042]
[0043] In a three-necked reaction flask, add 3-(4-chlorophenyl)glutaric acid (4.1mmol) and Ac 2 O (20mL), stirred, heated to 100°C, reacted for 2h, then concentrated to dryness to obtain an oil. Anhydrous tetrahydrofuran (20 mL), triethylamine (10.3 mmol) and 2-aminoindane hydrochloride (4.1 mmol) were added to the above oil, and stirred overnight at room temperature. After the reaction was complete, it was concentrated to dryness, added water (60 mL), extracted with ethyl acetate (30 mL×3), washed once with saturated brine, dried over anhydrous magnesium sulfate, filtered, and concentrated to dryness to obtain an oil. To the above oil, dichloromethane (30 mL), 2-(7-azabenzotriazole)-N,N,N',N'-tetramethyluronium hexafluorophosphate (HATU, 4.9 mmol), N,N-diisopropylethylamine (DIPEA, 8.2mmol) and 1-(2-aminoethyl)piperidine (4.9mmol), stirred overnight at room temperature. After the reaction is complete, adjus...
Embodiment 3
[0053] Embodiment 3: the preparation of compound 6-9
[0054]
[0055] In a three-necked reaction flask, add 3-phenylglutaric acid (4.8mmol) and Ac 2 O (20mL), stirred, heated to 100°C, reacted for 2h, concentrated to dryness to obtain an oil. Anhydrous tetrahydrofuran (20 mL), triethylamine (12.0 mmol) and 2-aminoindane hydrochloride (4.8 mmol) were added to the above oil, and stirred overnight at room temperature. After the reaction was complete, the reaction solution was concentrated to dryness, poured into water (60 mL), extracted with ethyl acetate (30 mL×3), washed once with saturated brine, dried over anhydrous magnesium sulfate, filtered, and concentrated to dryness to obtain an oil. To the above oil, dichloromethane (30 mL), 2-(7-azabenzotriazole)-N,N,N',N'-tetramethyluronium hexafluorophosphate (HATU, 5.8 mmol), N,N-diisopropylethylamine (DIPEA, 9.6mmol) and 1-(2-aminoethyl)piperidine (5.8mmol), stirred overnight at room temperature. After the reaction is compl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com